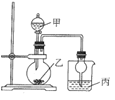
��Ŀ����
����Ŀ�������£���0.10 molL-1NH3H2O����Һ�ζ�20 mL0.10 molL-1������δ֪Ũ��CH3COOH�Ļ����Һ�������Һ����Ե��������仯������ͼ��ʾ����֪Kb(NH3��H2O) =Ka(CH3COOH)�����������������
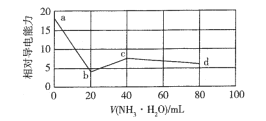
A.H+�ĵ����������ڵ�Ũ�ȵ�NH4+
B.a������Һ��c( CH3COO- ) +c( CH3COOH) =0.10 molL-1
C.b������Һ�У�c( NH4+ ) +c( NH3H2O) >c( CH3COOH)
D.c������Һ�У�c(NH4+) >c(Cl-) >c(CH3COO-) >c(OH-)>c(H+)
���𰸡�D
��������
����ͼ����Ϣ����20mL 0.10 molL1 NH3H2Oʱ����������ͣ����ʱ20 mL0.10 molL1����ǡ�÷�Ӧ�꣬�ټ���20mL 0.10 molL1 NH3H2Oʱ�����������ӵ�������˵����40mLʱCH3COOH�����꣬��˵��CH3COOH��Ũ��Ϊ0.10 molL1���ټ���NH3H2Oʱ���������½����������Һ�����������Ũ�ȼ�С��
A. a��������ʹ���Ļ����Һ��Ũ����ȣ�b��������շ�Ӧ��ȫ��b��������NH4Cl��CH3COOH�Ļ����Һ�����Դ���������Һ����Ӻ͵ı仯����Һ�����Ϊ40mL��b��NH4Cl��a������Ũ�ȵ�һ�룬���H+�ĵ����������ڵ�Ũ�ȵ�NH4+����b�㵼������Ӧ����a�㵼��������һ�룬��b�㵼��������a�㵼������һ�㻹С��˵��H+�ĵ����������ڵ�Ũ�ȵ�NH4+����A��ȷ��
B. ���ݷ���ԭ20mL��Һ��CH3COOH��Ũ��Ϊ0.10 molL1�����a������Һ��c(CH3COO��) +c( CH3COOH) =0.10 molL1����B��ȷ��
C. b������ΪNH4Cl��CH3COOH�Ļ����Һ������Ũ����ȣ����������غ㣬c(NH4+) +c(NH3H2O) = c(CH3COOH) + c(CH3COO��)�����c(NH4+) +c(NH3H2O)�� c(CH3COOH)����C��ȷ��
D. c������Һ����ΪNH4Cl��CH3COONH4��NH4+��CH3COO��ˮ�⣬����Kb(NH3��H2O) =Ka(CH3COOH)�����CH3COONH4�����ԣ�NH4Cl�����ԣ���˻����Һ�����ԣ���D����
������������ΪD��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�����������Դ�ǵ����о���һ���ȵ����⡣������(CH3OCH3)��δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�ã���ҵ����CO��H2Ϊԭ������CH3OCH3����ҵ�Ʊ��������ڴ���Ӧ����(ѹ��2.0��10.0Mpa���¶�230��280��)�������з�Ӧ��
��CO(g)+2H2(g)CH3OH(g) ��H1=-99kJ��mol1
��2CH3OH(g)CH3OCH3(g)+H2O(g) ��H2=-23.5kJ��mol1
��CO(g)+H2O(g)CO2(g)+H2(g) ��H3=-41.2kJ��mol1
(1)����Ӧ���е��ܷ�Ӧ3CO(g)+3H2(g)CH3OCH3(g)+CO2(g)��������H=_______����Ӧ����ú����������֪�÷�Ӧ��ƽ�ⳣ������ʽΪK=![]() ��ÿ����1mol H2��Ҫ����131.3kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ____________��
��ÿ����1mol H2��Ҫ����131.3kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ____________��
(2)�ڸ������£�����Ӧ�ٵ���ʼŨ�ȷֱ�Ϊ��c(CO)=0.6mol��L1��c(H2)=1.4mol��L1��8min��ﵽƽ�⣬CO��ת����Ϊ50%����8min��H2��ƽ����Ӧ����Ϊ__________��
(3)��t��ʱ����Ӧ�ڵ�ƽ�ⳣ��Ϊ400�����¶��£���1L���ܱ������м���һ���ļ״�����Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
c(mol��L1) | 0.46 | 1.0 | 1.0 |
��ʱ��v��___v��(���������������)��ƽ��ʱc(CH3OCH3)�����ʵ���Ũ����___��
(4)��(1)С���д���Ӧ�ҵ��ܷ�Ӧ3CO(g)+3H2(g)CH3OCH3(g)+CO2(g)��CO��ƽ��ת����a(CO)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��ͼ��X����___(��¶ȡ���ѹǿ��)����L1___L2(���������������)��
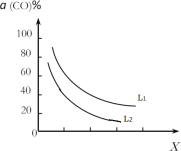
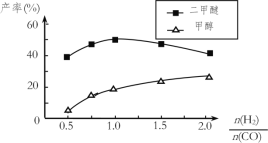
(5)�ڴ�����������ͬʱ����������Ӧ������������ʼͶ�ϱ�![]() �ĸı䣬�����Ѻͼ״��IJ���(�����е�̼ԭ��ռ��ʼCO��̼ԭ�ӵİٷ���)������ͼ�ı仯���ơ��Խ���Ͷ�ϱȴ���1.0֮������Ѳ��ʺͼ״����ʱ仯��ԭ��_____��
�ĸı䣬�����Ѻͼ״��IJ���(�����е�̼ԭ��ռ��ʼCO��̼ԭ�ӵİٷ���)������ͼ�ı仯���ơ��Խ���Ͷ�ϱȴ���1.0֮������Ѳ��ʺͼ״����ʱ仯��ԭ��_____��
����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���
ʵ�� | �Լ��� | �Լ��� | �Լ��� | ʵ����� |
|
A | Ũ���� | ͭƬ | ������KI��Һ | �����ԣ�NO2��I2 | |
B | ϡ���� | FeS | ��AgNO3��AgCl��Һ | Ksp(AgCl)��Ksp(Ag2S) | |
C | Ũ��ˮ | CaO | ��ɫʯ����Һ | ��ˮ�ʼ��� | |
D | ϡ���� | ʯ��ʯ | BaCl2��Һ | ��������BaCO3���� |
A.AB.BC.CD.D