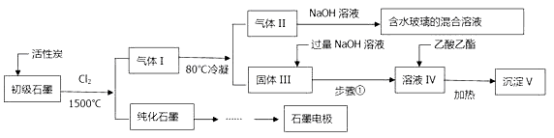
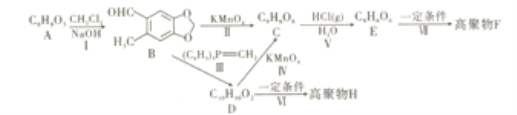
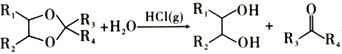��Ŀ����
����Ŀ��C��N��O��S��H������ѧ��ѧ�г�����Ԫ�ء���Ҫ�����������ա�
��1��0.5 mol CH4������Ϊ____________����״���£�4.48 L NH3����������Ϊ___________��
��2�������ʵ�����CH4��NH3��N2�������壬����ԭ����֮��Ϊ__________����������CH4��N2���ʵ���֮��Ϊ__________��
��3������1.806��1024�����ӵ�OH�� �����ʵ���Ϊ____��0.5 mol H2O������������������_____��NH4+��������������ȡ�
��4���ڱ�״���£���SO2��CO2��ɵĻ������Ϊ8.96 L������Ϊ24 g���û�������ƽ��Ħ��������__________�����������CO2�����������__________��
���𰸡�8.0 g 0.2 NA 5 : 4 : 2 7 : 4 0.3 mol 0.5 NA��3.01��1023 60 g/mol 20%
��������
��1��0.5 mol CH4������Ϊ0.5mol��16g/mol��8.0g����״���£�4.48 L NH3�����ʵ�����4.48L��22.4L/mol��0.2mol���������������Ϊ0.2NA��
��2��CH4��NH3��N2�������壬����ԭ�����ֱ���5��4��2��������ʵ�����CH4��NH3��N2�������壬����ԭ����֮��Ϊ5:4:2������m��nM��֪��������CH4��N2���ʵ���֮��Ϊ��Է�������֮�ȵķ��ȣ���Ϊ28��16��7��4��
��3������1.806��1024�����ӵ����ʵ�����![]() ��1�����������Ӻ���10�����ӣ���OH�������ʵ���Ϊ0.3mol��1����ˮ��1��笠�������10�����ӣ���0.5 mol H2O������������������0.5NA��NH4+��������������ȡ�
��1�����������Ӻ���10�����ӣ���OH�������ʵ���Ϊ0.3mol��1����ˮ��1��笠�������10�����ӣ���0.5 mol H2O������������������0.5NA��NH4+��������������ȡ�
��4���ڱ�״���£���SO2��CO2��ɵĻ������Ϊ8.96 L�����ʵ�����8.96L��22.4L/mol��0.4mol������Ϊ24 g���û�������ƽ��Ħ��������24g��0.4mol��60 g/mol������ʮ�ֽ��淨��֪SO2��CO2�����ʵ���֮����![]() �����Ի��������CO2�����������
�����Ի��������CO2�����������![]() ��
��

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�����Ŀ�����Ȼ�����(POCl3)�������л��ϳɵ��Ȼ����ʹ�����ʵ������ȡPOCl3���ⶨ��Ʒ������ʵ�����������
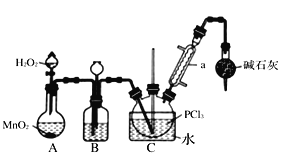
I���Ʊ�POCl3������������Һ̬��PCl3����ʵ��װ��(���ȼ��г�װ��ʡ��)�������Ϣ���¡�
���� | �۵�/�� | �е�/�� | ��Է������� | �� �� |
PCl3 | -112.0 | 76.0 | 137.5 | ��Ϊ��ɫҺ�壬��ˮ������ˮ��Ϊ��������Ȼ��⣬������ |
POCl3 | 2.0 | 106.0 | 153.5 |
��1��װMnO2������������__________������ܵ�����Ϊ_________________________��
��2��װ��B�е�Һ��ҩƷ��_____��װ��B���������ã��ֱ�Ϊ______________________��
��3�����Ȼ������������Ȼ�����ˮ��������Ӧ�Ʊ����˷����������Ȼ����Ļ�ѧ����ʽΪ____________________________________��
II���ⶨPOCl3��Ʒ������ʵ�鲽�裺
��ʵ��I��������Ӧ����Һ����ȴ�����£�ȷ��ȡһ�������� POCl3��Ʒ�����ʲ�����Ԫ�أ�������ʢ��100.00 mL����ˮ���ձ���ҡ������ȫˮ�⣬��ˮ��Һ���200.00 mL��Һ
��ȡ10.00 mL��Һ����ƿ�У�����10.00 mL 1.5 mol/L AgNO3����Һ
�ۼ�������������������ҡ��������
�ܼ���ָʾ������0.2 mol/L KSCN��Һ�ζ�������AgNO3��Һ�������յ�ʱ����ȥ15.00 mL KSCN��Һ��
��4��ѡ���ָʾ��Ϊ___________��
��5����ò�Ʒ��n(POCl3)= ___________________________��(��֪���������������ᣩ
��6����֪Ksp(AgCl)> Ksp(AgSCN)������۵�Ŀ����________________________________��