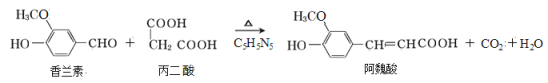��Ŀ����
����Ŀ����ҵ�ϳɰ���Ӧ�Ļ�ѧ����ʽΪ��N2(g)+3H2(g) ![]() 2NH3(g) ������92.3kJ/mol��һ���¶��£������Ϊ2L���ܱ������м���1mol N2��3mol H2����2min��ﵽƽ�⣬ƽ��ʱ���NH3��Ũ��Ϊ0.5 mol/L��
2NH3(g) ������92.3kJ/mol��һ���¶��£������Ϊ2L���ܱ������м���1mol N2��3mol H2����2min��ﵽƽ�⣬ƽ��ʱ���NH3��Ũ��Ϊ0.5 mol/L��
��1��2min ��H2�ķ�Ӧ����v(H2)�� ��
��2����ַ�Ӧ���ﵽƽ��ʱ���ų������� 92.3kJ����������������С������������������
ԭ���� ��
��3������˵����֤����Ӧ�ﵽƽ��״̬�� ��
A����λʱ���ڣ��Ͽ�1mol N��N��ͬʱ�Ͽ�3mol H��H
B����λʱ���ڣ��γ�1mol N��N��ͬʱ�γ�3mol N��H
C����λʱ���ڣ��Ͽ�1mol N��N��ͬʱ�Ͽ�6mol N��H
D����λʱ���ڣ��γ�1mol N��N��ͬʱ�Ͽ�3mol H��H
��4������һ����Ҫ��;�������Ʊ��������ԭ��N2H4����������֪����������ԭ����N2H4��������NO2��ȼ�գ�����N2��ˮ�������������·�Ӧ��
N2(g)+2O2(g)=2NO2(g) ��H1����67.7kJ/mol
N2H4(g)+O2(g)=N2(g)+2H2O(g) ��H2����534��0kJ/mol
д������ͬ״̬�£���������Ӧ���Ȼ�ѧ����ʽ ��
���𰸡���1��0.375 mol/(L��min)��
��2��С�ڣ� ���淴Ӧ���ն�̬ƽ�⣬ʼ�մﲻ�����ų�����
��3��C��D ��
��4��2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=-1135 .6 kJ��mol-1��
��������
�����������1��2min ��H2�ķ�Ӧ����v(NH3)��0.5 mol/L��2min=0.25 mol/(L��min)������v(H2)=3/2 v(NH3)��0.375 mol/(L��min)����2�����ڸ÷�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫת��Ϊ��������������Ϊ2L���ܱ������м���1mol N2��3mol H2����2min��ﵽƽ��ʱ���ų�������С��92.3kJ����3��A����λʱ���ڣ��Ͽ�1mol N��N��ͬʱ�Ͽ�3mol H��H��������Ӧ������У������ж�Ϊƽ��״̬������B����λʱ���ڣ��γ�1mol N��N��ͬʱ�γ�3mol N��H��������Ӧ������У�δ�ﵽƽ��״̬������C����λʱ���ڣ��Ͽ�1mol N��N��ͬʱ�Ͽ�6mol N��H����Ӧ�ﵽƽ��״̬����ȷ��D����λʱ���ڣ��γ�1mol N��N��ͬʱ�Ͽ�3mol H��H���������ʵ�Ũ�Ȳ��䣬��Ӧ�ﵽƽ��״̬����ȷ����4�����ݸ�˹���ɣ����ڶ���ʽ������2���ټ�ȥ��һ��ʽ�ӣ������ɵ�2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=-1135 .6 kJ��mol-1��

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�