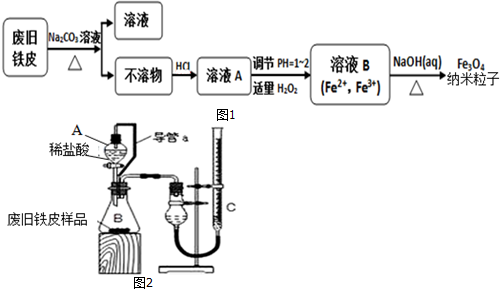
��Ŀ����
14��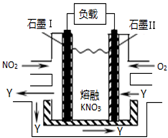 ��1��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ����ͼ���õ����ʹ�ù�����ʯīI�缫������������Y����缫��ӦʽΪNO2+NO3--e-�TN2O5��������1molY������Ҫ���ı�״�������������Ϊ5.6L��
��1��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ����ͼ���õ����ʹ�ù�����ʯīI�缫������������Y����缫��ӦʽΪNO2+NO3--e-�TN2O5��������1molY������Ҫ���ı�״�������������Ϊ5.6L����2��һ�Լ״�Ϊԭ�ϣ�ʹ�����Ե���ʹ���ȼ�ϵ�أ���ȼ�ϵ�صĸ�����ӦʽΪ2CH3OH+2H2O-12e-=2CO2+12H+�����Լ�������ȼ�ϵ���еļ״���������ṩ��ȵĵ�������ÿ����3.2g�״��������״���µļ�������Ϊ1.68��
���� ��1�������ڢ�缫�Ϸ���������Ӧ���缫���Ϸ�����ԭ��Ӧ��NO2���������ɵ�������ֻ����N2O5���ݵ����غ�����Ҫ�����������
��2���ܷ�ӦʽΪ2CH3OH+3O2��g��=2CO2��g��+4H2O�������ǻ�ԭ��Ӧ�������������ŵ磬���������»�õ�������ˮ���ܷ�Ӧʽ��ȥ������Ӧʽ�ɵø����缫��Ӧʽ������ת�Ƶ�����Ŀ��ȼ����������ʵ������ٸ���V=nVm���㣮
��� �⣺��1�������ڢ�缫�Ϸ���������Ӧ���缫���Ϸ�����ԭ��Ӧ��NO2���������ɵ�������ֻ����N2O5���缫���ϵĵ缫��ӦʽΪ��NO2+NO3--e-�TN2O5������1molN2O5��ʧȥ1mol���ӣ��õ�1mol������Ҫ0.25mol������0.25mol��22.4L/mol=5.6L��
�ʴ�Ϊ��NO2+NO3--e-�TN2O5��5.6��
��2���ܷ�ӦʽΪ2CH3OH+3O2��g��=2CO2��g��+4H2O�������ǻ�ԭ��Ӧ�������������ŵ磬���������»�õ�������ˮ�������缫��ӦʽΪ��3O2+12H++12e-=6H2O���ܷ�Ӧʽ��ȥ������Ӧʽ�ɵø����缫��ӦʽΪ��2CH3OH+2H2O-12e-=2CO2+12H+����CH3OH+H2O-6e-=CO2+6H+��3.2g�״������ʵ���Ϊ0.1mol����ȫȼ��ת�Ƶ�����Ϊ0.1mol��6=0.6mol������Ҫ��������ʵ���Ϊ$\frac{0.6}{8}$mol=0.075mol����Ҫ��������Ϊ0.075mol��22.4L/mol=1.68L��
�ʴ�Ϊ��CH3OH+H2O-6e-=CO2+6H+��1.68��
���� ���⿼����ȼ�ϵ�صĹ���ԭ���Լ�������ԭ��Ӧ����ʽ����д���缫��Ӧʽ��д�������غ��Ӧ�ã���Ŀ�Ѷ��еȣ�

| v��mol•L-1•s-1�� | c��H2����mol•L-1�� | c��Cl2����mol•L-1�� |
| 1.0k | 1.0 | 1.0 |
| 2.0k | 2.0 | 1.0 |
| 4.0k | 2.0 | 4.0 |
| A�� | m=1��n=1 | B�� | m=$\frac{1}{2}$��n=$\frac{1}{2}$ | C�� | m=$\frac{1}{2}$��n=1 | D�� | m=1��n=$\frac{1}{2}$ |
| A�� | ��pH�Ʋⶨ0.1mol/L��ˮ��pH | |
| B�� | ��pH��ֽ�ⶨ0.1mol/LNH4Cl��Һ��pH | |
| C�� | ����ˮ������һ�������ݵ�ͨ���·�У��۲�������� | |
| D�� | ��Ũ�ȡ�������İ�ˮ��NaOH�ֱ����Ũ�ȵ����ᷴӦ���Ƚ�������������Ķ��� |