��Ŀ����
����Ŀ���ҹ���ѧ�ҽ����������Ƶ������������Ͻ���������˿ɿؽṹ����̼���ܵ��Ʊ����⡣�������������ں�������ŵ�һ�����ɫ������̲��Ŵ����Ľ�����Դ�������١������̡�п���ܵȡ�
��1����̬��ԭ�ӵĺ���δ�ɶԵ�����Ϊ________������̼���ܿɿ���ʯīϩ��һ������������ɵĿ���Բ���壬��̼ԭ�ӵ��ӻ���ʽΪ________��
��2�����ṹ�����ܿ��������½�����ȩ![]() ��ȫ������������ȩ���ӵ�����ԭ�ӵ�VSEPR����Ϊ________���������
��ȫ������������ȩ���ӵ�����ԭ�ӵ�VSEPR����Ϊ________���������![]() ����
����![]() ���ĸ�����Ϊ________��
���ĸ�����Ϊ________��
��3�����ʻ���![]() ���۵�Ϊ
���۵�Ϊ![]() ����һ����Ҫ�����������������ڶ����л��ܼ����������Ԫ�صĵ�һ��������С�����˳��Ϊ________
����һ����Ҫ�����������������ڶ����л��ܼ����������Ԫ�صĵ�һ��������С�����˳��Ϊ________![]() ��Ԫ�ط���
��Ԫ�ط���![]() ������CO����W�γ���λ����ԭ����C��O��ԭ����________________________��
������CO����W�γ���λ����ԭ����C��O��ԭ����________________________��
��4����ԭ�ӷ����и�ԭ������ͬһƽ���ڣ������ƽ�е�p�������p���ӿ��ڶ��ԭ�Ӽ��˶����γ�������![]() ���������з����д���������
��������������������![]() ��������________
��������________![]() ����ĸ
����ĸ![]() ��
��
A.������ ![]() ��������
�������� ![]() ��������
�������� ![]() ����
����
��5��![]() ��
��![]() ����
����![]() ����γ������ӣ���ṹ��ͼ1��ʾ����������������ӿ��γɻ������Σ��ü��εĻ�ѧʽΪ________��
����γ������ӣ���ṹ��ͼ1��ʾ����������������ӿ��γɻ������Σ��ü��εĻ�ѧʽΪ________��
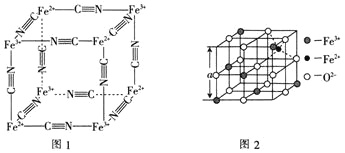
��6��ͼ2�Ǵ����������Ӿ���![]() ��ȡ�����������侧��ṹ��һ�������塣��֪
��ȡ�����������侧��ṹ��һ�������塣��֪![]() ������ܶ�Ϊ
������ܶ�Ϊ![]() ����ͼ2��
����ͼ2��![]() ________
________![]() ��֪
��֪![]() ��
��
���𰸡�![]()
![]() ƽ�������� 1��3
ƽ�������� 1��3 ![]() Cԭ�Ӱ뾶��O�縺�Խ�С���Թ¶Ե��ӵ��������������������γ���λ��
Cԭ�Ӱ뾶��O�縺�Խ�С���Թ¶Ե��ӵ��������������������γ���λ�� ![]()
![]()
![]()
��������
��1����ɸ������ǵ���̼��������ʯīϩ�����γɵģ�ʯīϩ�������ʯī��ʯīÿһ���� ƽ��ṹ������Զ�����
ƽ��ṹ������Զ�����![]() �ӻ���
�ӻ���
��2����������ͬ��Ԫ�������������˼���ȩ��ȫ�����ռ�ȩ��������
��3����һ�����ܿ�����ʧ������������ԭ�ԣ���ǿ�����жϣ�����һ������Խ��Խ����ʧ���ӣ�������ԭ�����ж��ԭ�Ӿ����ṩ�¶Ե��ӣ���˭�Թ¶Ե��ӵ�������������˭������ԭ�ӣ�
��4�����ݷ��ӵĽṹ��������治�����ƽ�е�p����������жϴ治����������![]() ������
������
��5�������ϵ�ԭ�Ӱ�![]() ���㣬ÿ�����м��ԭ�Ӱ�
���㣬ÿ�����м��ԭ�Ӱ�![]() ���㼴�ɣ�ע������
���㼴�ɣ�ע������![]() ��
��![]() ��
��
��6����Ŀ���ܶ���֪�����ֻҪ����![]() ���������ʽ���ɣ�ע�ⵥλ�Ļ��㡣
���������ʽ���ɣ�ע�ⵥλ�Ļ��㡣
![]() ���ݺ��ع���ԭ�ӵļ۵����Ų�ʽΪ
���ݺ��ع���ԭ�ӵļ۵����Ų�ʽΪ![]() ����û���γɵ��Ӷԣ�����δ�ɶԵ�����Ϊ6��ʯīϩ��ƽ��ṹ�����Ը�̼ԭ�ӵ��ӻ���ʽΪ
����û���γɵ��Ӷԣ�����δ�ɶԵ�����Ϊ6��ʯīϩ��ƽ��ṹ�����Ը�̼ԭ�ӵ��ӻ���ʽΪ![]() ��
��
![]() ���ݼ۲���ӶԻ������۵�֪
���ݼ۲���ӶԻ������۵�֪![]() ��������ԭ����
��������ԭ����![]() �ӻ�������ԭ�ӵ�VSEPR����Ϊƽ�������Σ��������
�ӻ�������ԭ�ӵ�VSEPR����Ϊƽ�������Σ��������![]() ����
����![]() ������Ŀ�ֱ���1��3��
������Ŀ�ֱ���1��3��
![]() ����������Ϊ2����C��O��ԭ�ӵĵ�һ������ҪС��C��O��ԭ�ӵĺ�������Ų�ʽ�ֱ�Ϊ
����������Ϊ2����C��O��ԭ�ӵĵ�һ������ҪС��C��O��ԭ�ӵĺ�������Ų�ʽ�ֱ�Ϊ![]() ��
��![]() ������Oԭ�ӵĵ�һ�����ܸ��������ʻ���
������Oԭ�ӵĵ�һ�����ܸ��������ʻ���![]() �����У�W������ԭ�ӣ�CO�����壬����Cԭ�Ӱ뾶��O�縺��С���Թ¶Ե��ӵ��������������������γ���λ������������CO����W�γ���λ��ԭ����C��O��
�����У�W������ԭ�ӣ�CO�����壬����Cԭ�Ӱ뾶��O�縺��С���Թ¶Ե��ӵ��������������������γ���λ������������CO����W�γ���λ��ԭ����C��O��
![]() ��ԭ�ӷ����и�ԭ������ͬһƽ���ڣ������ƽ�е�p�������p���ӿ��ڶ��ԭ�Ӽ��˶����γ�������
��ԭ�ӷ����и�ԭ������ͬһƽ���ڣ������ƽ�е�p�������p���ӿ��ڶ��ԭ�Ӽ��˶����γ�������![]() ������
������
A.�����������ֻ����![]() ����������
����������![]() ������A����
������A����
B.������������е�����ԭ��S������Oԭ�������ƽ�е�p����������γ�������![]() ��������������
��������������![]() ����Ϊ
����Ϊ![]() ����B��ȷ��
����B��ȷ��
C.��������������ֻ����![]() ����������
����������![]() ������C����
������C����
D.���ӷ����еı����ϵ�Cԭ�����ǻ�Oԭ�������ƽ�е�p����������γ�������![]() ��������������
��������������![]() ����Ϊ
����Ϊ![]() ����D��ȷ��
����D��ȷ��
��ѡBD��
![]() ����ͼ1���Ӿ����ṹ������
����ͼ1���Ӿ����ṹ������![]() ��
��![]() ����
����![]() ��Ϻ��γɵ�����Ϊ
��Ϻ��γɵ�����Ϊ![]() �����Ը������γɵļ��εĻ�ѧʽΪ
�����Ը������γɵļ��εĻ�ѧʽΪ![]() ����
����![]() �����дΪ
�����дΪ![]() ��
��
![]() ͼ2��
ͼ2��![]() ����ĿΪ
����ĿΪ![]() ��
��![]() λ���������ڣ���ĿΪ1�������ӵ���ĿΪ
λ���������ڣ���ĿΪ1�������ӵ���ĿΪ![]() �������ܶ�
�������ܶ�![]() �ɵ�
�ɵ�![]() �������
�������![]() ��
��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����. (1) ��֪S4�Ľṹʽ��ͼ����ӦS4(g) + 4Cl2(s )== 4SCl2(g) ��H= - 4 kJ��mol-1��S��S���ļ���Ϊ266 kJ��mol-1��S��Cl���ļ���Ϊ255 kJ��mol-1����1mol Cl2(g)�����еĻ�ѧ������ʱ��Ҫ���յ�����Ϊ_____kJ��
��. ��ҵ�Ϻϳ��Ȼ�������Ӧ��SO2(g)+ SCl2(g)+Cl2(s)![]() 2SOCl2(g)���÷�Ӧ��ijһ��Ӧ����������(��A%��ʾ)���¶ȵı仯��ϵ��ͼ��ʾ��
2SOCl2(g)���÷�Ӧ��ijһ��Ӧ����������(��A%��ʾ)���¶ȵı仯��ϵ��ͼ��ʾ��
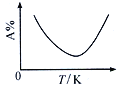
(2) ��373Kʱ����2L�ܱ�������ͨ�����ʵ�����Ϊ0.04 mol��SO2��SCl2��Cl2�� ����������Ӧ�������ѹǿ(p)��ʱ��(t)�ı仯Ϊ�±������ݢ�(ƽ��ʱ���¶�����ʼ�¶���ͬ)
t/min | 0 | 1 | 2 | 3 | 4 | 5 |
�� | 6.0p0 | 6.7 p0 | 6.1 p0 | 5.4 p0 | 5.0 p0 | 5.0 p0 |
�� | 6.0 p0 | 7.0 p0 | 5.3 p0 | 5.0 p0 | 5.0 p0 | 5.0 p0 |
�ٸ÷�Ӧ�ġ�H_____0(������������������=��)��
�ڷ�Ӧ��ʼ���ﵽƽ��ʱ��v(SCl2)=__________��
����ֻ�ı�ijһ�����������ѹǿ��ʱ��ı仯Ϊ�������ݢ���ı��������_______________��
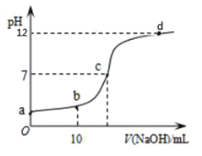
(3) ��ͼ��ijͬѧ�ⶨ��������Ӧ��pK(pK= - lgK)���¶ȵı仯��ϵͼ��

�� A�����ֵΪ_________(��֪lg4=0.6)��
�ڵ����ߵ�ijһ�¶�ʱ����Ӧ���´ﵽƽ�⣬A����ܱ仯Ϊ______�㡣
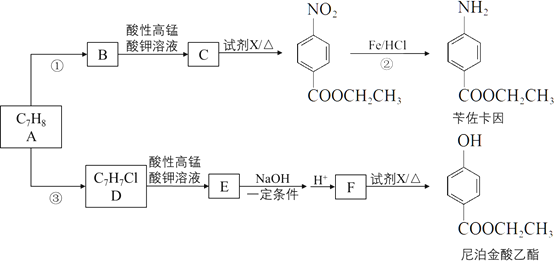
III. (4) ���NO2�Ʊ�NH4NO3���乤��ԭ����ͼ��ʾ��
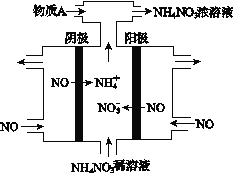
�������ĵ缫��ӦʽΪ_________________________��
��Ϊʹ������ȫ��ת��ΪNH4NO3���貹��ij������A����A�Ļ�ѧʽΪ___________��
����Ŀ��������������Ӱ�����ǵ�����ͽ�����������Ҫ��Ⱦ��Ϊ�����������PM2.5������Ҫ��ԴΪȼú��������β���ȡ���˸�����Դ�ṹ���������ŵȴ�ʩ����Ч����PM2.5��SO2��NOx����Ⱦ��
��ش��������⣺
��1������β����NOx��CO�����ɣ���֪����������NO�ķ�ӦΪ��N2(g)��O2(g)![]() 2NO(g)��Q�����¡������ܱ������У�����˵���У���˵���÷�Ӧ�ﵽ��ѧƽ��״̬����____��
2NO(g)��Q�����¡������ܱ������У�����˵���У���˵���÷�Ӧ�ﵽ��ѧƽ��״̬����____��
A.���������ܶȲ��ٱ仯
B.��������ѹǿ���ٱ仯
C.N2��O2��NO�����ʵ���֮��Ϊ1��1��2
D.������ת���ʲ��ٱ仯
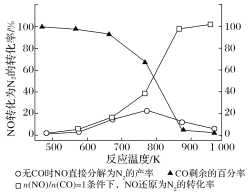
��2������ʹ���Ҵ����Ͳ����ܼ���NOx���ŷţ���ʹNOx����Ч������Ϊ�����������Ҫ���⡣ij�о���С����ʵ������Ag��ZSM��5Ϊ���������NOת��ΪN2��ת�������¶ȱ仯�����ͼ��ʾ������ʹ��CO���¶ȳ���775 K������NO�ķֽ��ʽ��ͣ�����ܵ�ԭ��Ϊ___����![]() ��1�������£�Ϊ���õij�ȥNOx��Ӧ���Ƶ�����¶���___K���ҡ�
��1�������£�Ϊ���õij�ȥNOx��Ӧ���Ƶ�����¶���___K���ҡ�
��3�������ŷŵĵ������úȼ�ղ����Ķ��������ǵ������������������������������̿�ɴ���������Ⱦ��NO����5L�ܱ������м���NO�ͻ���̿(����������)��һ����������������E��F�����¶ȷֱ���T1���T2��ʱ����ø�����ƽ��ʱ���ʵ���(n/mol)���±���
���� �¶�(��) | ����̿ | NO | E | F |
��ʼ | 3.000 | 0.10 | 0 | 0 |
T1 | 2.960 | 0.020 | 0.040 | 0.040 |
T2 | 2.975 | 0.050 | 0.025 | 0.025 |
��д��NO�����̿��Ӧ�Ļ�ѧ����ʽ��___��
����T1��T2����÷�Ӧ��Q__0(����������������������)��
��������ӦT1��ʱ�ﵽ��ѧƽ�����ͨ��0.1molNO���壬��ﵽ�»�ѧƽ��ʱNO��ת����Ϊ___��