��Ŀ����
����Ŀ��������������Ҫ�Ĺ�ҵԭ�ϣ�̽�����Ʊ����������ʾ��зdz���Ҫ�����塣
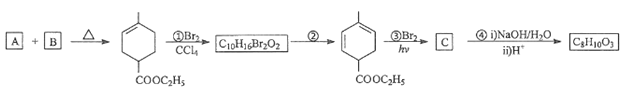
(1)��ҵ���û�����(FeS2��������Ԫ��Ϊ��1��)�ڸ����º�������Ӧ�Ʊ�SO2��
4FeS2��11O2![]() 8SO2��2Fe2O3���÷�Ӧ�б�������Ԫ����________(��Ԫ�ط���)�����÷�Ӧת��2.75 mol����ʱ�����ɵĶ��������ڱ�״���µ����Ϊ________ L��
8SO2��2Fe2O3���÷�Ӧ�б�������Ԫ����________(��Ԫ�ط���)�����÷�Ӧת��2.75 mol����ʱ�����ɵĶ��������ڱ�״���µ����Ϊ________ L��
(2)ʵ������������װ�òⶨSO2������ΪSO3��ת���ʡ�����֪SO3���۵�Ϊ16.8 �棬�����������װ��ʱ�ֱ���ȫ���գ��Һ��Կ�����CO2��Ӱ�죩
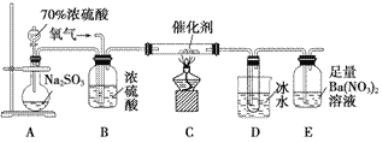
�ټ���ʹ�÷�Һ©����Բ����ƿ�еμ�Ũ����IJ�����__________________��
��ʵ������У���Ҫͨ����������д��һ��������ͼ��ʾװ����ȡ�����Ļ�ѧ����ʽ��________________________________________________________________________��
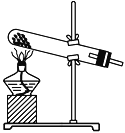
�۵�ֹͣͨ��SO2��Ϩ��ƾ��ƺ���Ҫ����ͨһ��ʱ�����������Ŀ����________________________________________________________________________��
��ʵ���������װ��D���ӵ�����Ϊm g��װ��E�в�����ɫ����������Ϊn g����������¶��������ת������________(�ú���ĸ�Ĵ���ʽ��ʾ�����û���)��
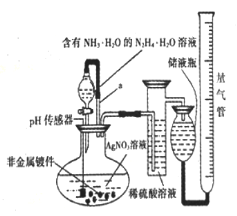
(3)ijѧϰС���������ͼװ����֤��������Ļ�ѧ���ʡ�
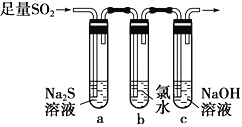
����˵������������������Ե�ʵ������Ϊ___________________________________��
��Ϊ��֤��������Ļ�ԭ�ԣ���ַ�Ӧ��ȡ�Թ�b�е���Һ�ֳ����ݣ��ֱ��������ʵ�顣
���������һ����Һ�м���AgNO3��Һ���а�ɫ��������
��������ڶ�����Һ�м���Ʒ����Һ����ɫ��ȥ
���������������Һ�м���BaCl2��Һ��������ɫ����
���������к�������________(�������)���Թ�b�з�����Ӧ�����ӷ���ʽΪ________________________________________________________________��
���𰸡� Fe��S 11.2 ��Һ©���ϿڵĻ�����������Һ©���������������μ� 2KClO3![]() 2KCl��3O2��(��2KMnO4
2KCl��3O2��(��2KMnO4![]() K2MnO4��MnO2��O2��) ʹ������װ���е�SO2��SO3���������
K2MnO4��MnO2��O2��) ʹ������װ���е�SO2��SO3��������� 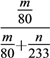 ��100% �Թ�a�г��ֵ���ɫ���� �� SO2��Cl2��2H2O===4H����SO
��100% �Թ�a�г��ֵ���ɫ���� �� SO2��Cl2��2H2O===4H����SO![]() ��2Cl��
��2Cl��
����������1��������ԭ��Ӧ4FeS2��11O2![]() 8SO2��2Fe2O3�У����ϼ����ߵ�Fe��SԪ���ڷ�Ӧ�б��������÷�Ӧ����8mol�Ķ�������ת�Ƶ��ӵ����ʵ���Ϊ44mol�����Ե��÷�Ӧת��2.75mol����ʱ�����ɵĶ�����������ʵ���Ϊ0.5mol���ڱ�״���µ����Ϊ11.2L����2����ʹ�÷�Һ©����Բ����ƿ�еμ�Ũ����IJ����Ǵ�Һ©���ϿڵĻ�����������Һ©���������������μӣ�����ͼ2��ʾװ����ȡ�����������ȹ���ķ�����������������������ڶ������̴������ȷֽ�������������ʽΪ��2KClO3
8SO2��2Fe2O3�У����ϼ����ߵ�Fe��SԪ���ڷ�Ӧ�б��������÷�Ӧ����8mol�Ķ�������ת�Ƶ��ӵ����ʵ���Ϊ44mol�����Ե��÷�Ӧת��2.75mol����ʱ�����ɵĶ�����������ʵ���Ϊ0.5mol���ڱ�״���µ����Ϊ11.2L����2����ʹ�÷�Һ©����Բ����ƿ�еμ�Ũ����IJ����Ǵ�Һ©���ϿڵĻ�����������Һ©���������������μӣ�����ͼ2��ʾװ����ȡ�����������ȹ���ķ�����������������������ڶ������̴������ȷֽ�������������ʽΪ��2KClO3![]() 2KCl��3O2������Ϊȷ��ʵ���ȷ�ȣ�Ҫ��֤���������������������IJⶨȷ����ֹͣͨ��SO2��Ϩ��ƾ��ƺ���Ҫ����ͨһ��ʱ���������������װ���еĶ�����������������ֱ������װ�����գ���װ��D���ӵ�����Ϊmg�����������������������mg�����ʵ�����mg��80g/mol��װ��E�в�����ɫ����������Ϊng�������յĶ�����������ʵ�����ng��233g/mol������������ת����=
2KCl��3O2������Ϊȷ��ʵ���ȷ�ȣ�Ҫ��֤���������������������IJⶨȷ����ֹͣͨ��SO2��Ϩ��ƾ��ƺ���Ҫ����ͨһ��ʱ���������������װ���еĶ�����������������ֱ������װ�����գ���װ��D���ӵ�����Ϊmg�����������������������mg�����ʵ�����mg��80g/mol��װ��E�в�����ɫ����������Ϊng�������յĶ�����������ʵ�����ng��233g/mol������������ת����=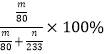 ����3���ٶ���������������ԣ����Ժ���ͼ۵����Ʒ���������ԭ��Ӧ�����ɵ���ɫ�ij���S�����ʵ������Ϊ���Թ�a�г��ֵ���ɫ���ǣ��ڷ���I�����һ����Һ�м���AgNO3��Һ���а�ɫ�������ɣ���������ˮ�е������Ӳ��������ã�����������ڶ�����Һ����Ʒ����Һ����ɫ��ȥ����������ˮ�к��е�Ư�������ʴ�����������ã������������������Һ����BaCl2��Һ��������ɫ������֤����Һ�к�����������ӣ��Ƕ������������Ի����º���ˮ��Ӧ���ɵģ���SO2+Cl2+2H2O��4H++SO42-+2Cl-��
����3���ٶ���������������ԣ����Ժ���ͼ۵����Ʒ���������ԭ��Ӧ�����ɵ���ɫ�ij���S�����ʵ������Ϊ���Թ�a�г��ֵ���ɫ���ǣ��ڷ���I�����һ����Һ�м���AgNO3��Һ���а�ɫ�������ɣ���������ˮ�е������Ӳ��������ã�����������ڶ�����Һ����Ʒ����Һ����ɫ��ȥ����������ˮ�к��е�Ư�������ʴ�����������ã������������������Һ����BaCl2��Һ��������ɫ������֤����Һ�к�����������ӣ��Ƕ������������Ի����º���ˮ��Ӧ���ɵģ���SO2+Cl2+2H2O��4H++SO42-+2Cl-��

 �Ķ��쳵ϵ�д�
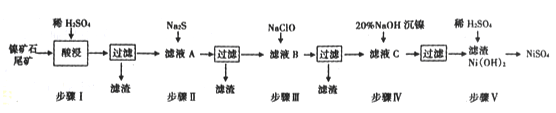
�Ķ��쳵ϵ�д�����Ŀ��������ʯβ������ȡNiSO4�ǽ���ҹ�����Դ�ѷ���һ����Ҫ;������֪�ù������£�
��1�������ʵ�Ksp�������±���
���� | MnS | NiS | PbS | CuS | Ni(OH)2 |
Ksp | 2.5��10-13 | 1.1��10-21 | 8.0��10-28 | 6.3��10-36 | 2.0��10-15 |
��2����ҺA�и��������ӵĺ������±���
�ɷ� | Ni2+ | Fe3+ | Fe2+ | Mn2+ | Cu2+ | Pb2+ | ���� |
3.80 | 4.80 | x | 0. 20 | 0.15 | <0.001 | ���� |
����������Ϣ���ش��������⣺
(1)����I���֮ǰ�轫��ʯ���飬Ŀ����____________��
(2)���������ӵ�Ũ��c��1.0��10-5mol/L�����϶�������ȫ��������е�Pb2+��ǡ�ó�����ȫʱ����Һ�������ӵ�Ũ��c(S2-)=_____________
(3)�����½��в�����Ŀ����Ϊ�˳�ȥ������Ԫ�أ���֪����Ԫ�ص����ӷ�Ӧ���£�
2Fe2++ ClO - +5H2O=2Fe( OH)3��+Cl-+4H+
��ʱMn2+����������ΪMnO2��д������Ԫ�ص����ӷ���ʽ______________��
(4)Ϊ�ⶨ��ҺA��Fe2+���ӵĺ�������ÿ����Һ�к��е��������ӵ���������g/L��ʾ��ÿ����ȡ20.00 mL����Һ������0.02 mol/L��KMnO4��Һ�ζ�������֪�������Ӿ�����Ӧ�������εζ�ƽ������KMnO4��Һ18. 00mL����x��ֵΪ________����ȷ��С�������λ����
��5������Ni(OH)2���������ӵ�ص���Ҫԭ�ϣ����ӵ�ع���ԭ�����£�
Cd+2NiO(OH) +2H2O![]() Cd(OH)2+2Ni(OH)2
Cd(OH)2+2Ni(OH)2
�����ŷŵ�Ľ��У�������pH______�����������С�����䡱�������ʱ�����缫��ӦʽΪ______��