��Ŀ����
����Ŀ��
I.��ͼ��ʾΪ����A�Ľṹ����֪����A����һ��Ԫ��X��ɡ�X��һ�ֵ��ʿ��ɽ���þ��XY2������ȷ�Ӧ��á���ش��������⣺

��1������A������Ϊ__________��
��2������A��Xԭ�ӵ��ӻ���ʽΪ________��
��3��ÿ��Aԭ�Ӳ����γ�______��6Ԫ����
��4����ÿ��Xԭ����Ϊһ������Xԭ�ӵİ뾶ΪR��1��������Xԭ�ӵ������ΪV����һ�����������ΪV0����������ϵ����=![]() ����þ���Ķѻ�ϵ����=_____������1λ��Ч���֣�ȡ
����þ���Ķѻ�ϵ����=_____������1λ��Ч���֣�ȡ![]() ���С�3��
����3��
����ʾ��ͼ�м�ͷ��ǵ�����ԭ�������еģ�����ԭ�����ķֱ�λ�������徧������������徧����Խ����ĵȷֵ㣩
II.�ɷ��ӹ��ײ�õĶ���1����ѧ�������������Ϊ�������ܣ�D0����1����ѧ��������ԭ��������ܳ�Ϊƽ������ܣ�D1�������ߵĹ�ϵΪ![]()
ʵ����X-X����Ƶ����=2��1014Hz��
���ʿ˳���h=6��10-34J��s
����٤������NA=6��1023mol-1
XΪ���׳�����ƽ������ܵļ���ʽΪ![]() ��
��
��ʵ����D1=6��10-19J��
�ð����ӵ�����NA��D0�õ�����1mol��ѧ�����������E��E��Ϊ���ܣ�E=NAD0��
��ش��������⣺
��1��X-X���ļ���Ϊ______kJ/mol��
��2��1mol����A����_____molX-X����
��3��ԭ�ӻ��ȵĶ���Ϊ������lmol���������л�ѧ����Ҫ���յ�������ԭ�Ӿ����ԭ�ӻ������������Ӿ���ľ����ܣ���ô����A��ԭ�ӻ���Ϊ______kJ/mol��
���𰸡� ���ʯ sp3 12 0.3 324.9 2 649.8
��������I.��1����������A���������ж�����Ϊ���ʯ����2�����ʯ��̼ԭ���γ�4�����������̼ԭ�ӵ��ӻ���ʽΪsp3�ӻ�����3�����ʯ������2��C-C�������γ�2����Ԫ����ÿ��6Ԫ������6��̼ԭ�ӣ���ÿ��̼ԭ�Ӳ����γ�12��6Ԫ������4����ÿ��Cԭ����Ϊһ������Cԭ�ӵİ뾶ΪR������������Խ�����8R���������߳���![]() ��������̼ԭ�Ӹ�4+6��1/2+8��1/2=8�����Զ����ϵ����=
��������̼ԭ�Ӹ�4+6��1/2+8��1/2=8�����Զ����ϵ����=![]() ��
��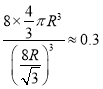 ��
��
I.��1��ƽ������ܵļ���ʽΪ![]() ����x��
����x��![]() ������
������![]() ������C��C��������NAD0��
������C��C��������NAD0�� ������������ݵõ�E��324.9kJ/mol����2��һ��̼ԭ���γ�2��C��C������1mol����A����2molC��C������3������1mol̼ԭ���γ�2��̼̼���������Ծ���A��ԭ�ӻ���Ϊ324.9kJ/mol��2��649.8kJ/mol��
������������ݵõ�E��324.9kJ/mol����2��һ��̼ԭ���γ�2��C��C������1mol����A����2molC��C������3������1mol̼ԭ���γ�2��̼̼���������Ծ���A��ԭ�ӻ���Ϊ324.9kJ/mol��2��649.8kJ/mol��

 ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�����Ŀ���±������ʵķ��������ȫ��ȷ����( )
���� | A | B | C | D |
ǿ����� | NaCl | H2SO4 | CaCO3 | H2S |
������� | HF | BaSO4 | HClO | NH3��H2O |
�ǵ���� | Cl2 | CS2 | CH4 | ���� |
A. AB. BC. CD. D