��Ŀ����
�����ߡ������־���ҹ��ĺ�����ҵ�������µ�ƪ�¡�
��1���������ʱ�������������ľ���Ħ�����������¡�Ϊ�˷�ֹ����¶ȹ��ߣ��ڻ��һ��Ϳ��һ�������Ϳ�ϣ���Ϳ�ϵ���������ܵ��� ��
��2�����������Ҫ���ܵ�ȼ�ϣ���������N2O4��N2H4����Ϊȼ�ϣ��䷴Ӧ�ķ���ʽ�ǣ�N2O4+N2H4��N2+H2O������ƽ�÷�Ӧ����ʽ�� N2O4+ N2H4�� N2+ H2O
�÷�Ӧ�б�������ԭ���뱻��ԭ��ԭ�ӵ����ʵ���֮���� �������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
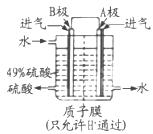
��3����ͼ��ij�ռ�վ����ת��ϵͳ�ľֲ�ʾ��ͼ������ȼ�ϵ�ز���KOHΪ���Һ��ȼ�ϵ�طŵ�ʱ�ĸ�����ӦΪ�� �����ij��ʱ�������������й��ռ���33.6L���壨������ɱ��������ö�ʱ����ˮ���ϵͳ��ת�Ƶ��ӵ����ʵ���Ϊ mol��

��4�������˺���������̬ϵͳ�У�����Ҫ�����ȥ��CO2����Ҫ���ṩ�����O2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2CO2=2CO+O2��CO������ȼ�ϡ� ��֪�÷�Ӧ��������ӦΪ��4OH��4e-=O2��+2H2O ��������ӦΪ�� �����������������Ʒ�Ӧ2CO=2C+O2����H��0����S��0��������CO����Ⱦ�������ж�������Ӧ�Ƿ��ܷ����� ���� ��
��5��.�������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ(C3H6)����������ɵñ�ϩ��
��֪����C3H8(g) CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1
CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1
��CH3CH=CH2(g) CH4(g)+ HC��CH (g)����H2="32.4" kJ��mol-1
CH4(g)+ HC��CH (g)����H2="32.4" kJ��mol-1
����ͬ�����£���ӦC3H8(g) CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1
CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1
��1���������ʱ�������������ľ���Ħ�����������¡�Ϊ�˷�ֹ����¶ȹ��ߣ��ڻ��һ��Ϳ��һ�������Ϳ�ϣ���Ϳ�ϵ���������ܵ��� ��
| A���ڸ����²��ڻ� | B���ڸ����¿ɷֽ����� |
| C���ڳ����¾ͷֽ����� | D����Ϳ�ϲ����ܷ����ֽ� |
�÷�Ӧ�б�������ԭ���뱻��ԭ��ԭ�ӵ����ʵ���֮���� �������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
��3����ͼ��ij�ռ�վ����ת��ϵͳ�ľֲ�ʾ��ͼ������ȼ�ϵ�ز���KOHΪ���Һ��ȼ�ϵ�طŵ�ʱ�ĸ�����ӦΪ�� �����ij��ʱ�������������й��ռ���33.6L���壨������ɱ��������ö�ʱ����ˮ���ϵͳ��ת�Ƶ��ӵ����ʵ���Ϊ mol��

��4�������˺���������̬ϵͳ�У�����Ҫ�����ȥ��CO2����Ҫ���ṩ�����O2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2CO2=2CO+O2��CO������ȼ�ϡ� ��֪�÷�Ӧ��������ӦΪ��4OH��4e-=O2��+2H2O ��������ӦΪ�� �����������������Ʒ�Ӧ2CO=2C+O2����H��0����S��0��������CO����Ⱦ�������ж�������Ӧ�Ƿ��ܷ����� ���� ��
��5��.�������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ(C3H6)����������ɵñ�ϩ��
��֪����C3H8(g)
 CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1
CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1��CH3CH=CH2(g)
 CH4(g)+ HC��CH (g)����H2="32.4" kJ��mol-1
CH4(g)+ HC��CH (g)����H2="32.4" kJ��mol-1����ͬ�����£���ӦC3H8(g)
 CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1
CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1��1��B ��2��1 2 3 4 ��2��1 ��������Ⱦ��3��H2-2e-+2OH-=2H2O 2
��4��2CO2+4e-+2H2O=2CO+4OH- ������ ��H>0������ ��S<0 ��5��(124.2)
��4��2CO2+4e-+2H2O=2CO+4OH- ������ ��H>0������ ��S<0 ��5��(124.2)
�����������Ϳ�ϵ���������ܵ����ڸ����¿ɷֽ��������ֽ����ȣ���������Ҳ���Ĵ���������������ʹ����¶Ƚ��͡�ѡ��Ϊ��B����2�����������Ҫ�������ܵķ�Ӧ�ķ���ʽ��1N2O4+2N2H4==3N2+4H2O��ϵ����1 2 3 4���÷�Ӧ�б�����N2H4�е�N��ԭ���뱻��ԭ��ԭ�ӵ�N2O4�е�Nԭ�ӵ����ʵ���֮����2:1. �����ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��Dz�������Ⱦ���ྻ����������3��������ȼ�ϵ���У�ͨ��H2�ĵ缫�������������ĵ缫��ӦΪ��H2-2e-+2OH-=2H2O. 2H2O------2H2��+ O2������������3mol,ת�Ƶ���4mol.���ڲ������干1.5mol������ת�Ƶ���2mol.��4�������ܷ�Ӧʽ�������ĵ缫��Ӧʽ�ɵõ�������ӦΪ:2CO2+4e-+2H2O=2CO+4OH- �������2CO=2C+O2����H��0����S��0��������CO����Ⱦ�ķ��������С�ԭ���Ǹ÷�Ӧ�ġ�H>0�������ȷ�Ӧ����S<0����ϵ�Ļ��ҳ̶ȼ�С����5����-�������ã�C3H8(g)
 CH3CH=CH2(g)+H2(g)�ġ�H=124.2KJ/mol.
CH3CH=CH2(g)+H2(g)�ġ�H=124.2KJ/mol.
��ϰ��ϵ�д�
�����Ŀ
 mol
mol mol
mol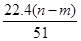 L
L ��
��
 2SO3����һ�ܱ�������һ��ʱ���ڴﵽƽ�⡣
2SO3����һ�ܱ�������һ��ʱ���ڴﵽƽ�⡣