��Ŀ����
��������ˮ��Һ�׳�˫��ˮ���е��ˮ�ߣ����⡢�ȼ��ؽ���������Ⱦ�������ֽ⡣
��1��ij�Լ������Ƶ�7%~8%��˫��ˮ��������Ũ����30%����Һ�����˷�����
����д��ţ���
a����ѹ���� �� b����ѹ���� c��������ʯ�ҳ�ѹ���� ���� d����ѹ����
��2������õ���˫��ˮ����Ԫ�صĺ���Ϊ90%�����������Ĵ���Ϊ ��������֪�������ڿ�����ȼ������ˮ����������������ڿ�����ȼ��Ҳ��������H2O2����������¶��ֽ��ˡ�Ϊ����֤�����ڿ�����ȼ�յIJ������Ƿ���H2O2��ij����С��ͬѧ��Ƶ�ʵ��װ�ü�ͼ-1��

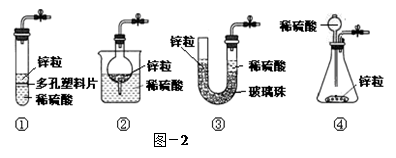
��3����ͬѧ�����ͼ-2�Ģ٣�����ѡȡ���ͼ��1�����е�װ�ã����е��� ����д��ţ���
��4������ͬѧ�����Ը��������Һ����H2O2�Ĵ��ڣ���ɸ÷�Ӧ�����ӷ���ʽ��
�� + �� Mn2+ + H2O
��ͬѧ���ҵļ��鷽����������ɣ���п����ϡ����ķ�Ӧ�в���������H2S�Ȼ�ԭ�����壬Ҳ��ʹ���Ը��������Һ��ɫ�������ͬѧ��ʵ�鷽������Ľ����飺 ��
��5����̼���ƣ�2Na2CO3?3H2O2���׳ƹ���˫��ˮ�����ֽ⣬��ֽⷴӦ�Ļ�ѧ����ʽ�ɱ�ʾΪ��2 (2Na2CO3?3H2O2) �� 4Na2CO3 + 6H2O + 3O2��
ȡһ�����Ĺ�̼�������ܱ�������ʹ����ȫ�ֽ⣬�����������12.0g����ȴ�����º������ò����м�ˮ���Ƴ�10.6% ��Na2CO3��Һ�����ˮ g��
��1��ij�Լ������Ƶ�7%~8%��˫��ˮ��������Ũ����30%����Һ�����˷�����
����д��ţ���
a����ѹ���� �� b����ѹ���� c��������ʯ�ҳ�ѹ���� ���� d����ѹ����
��2������õ���˫��ˮ����Ԫ�صĺ���Ϊ90%�����������Ĵ���Ϊ ��������֪�������ڿ�����ȼ������ˮ����������������ڿ�����ȼ��Ҳ��������H2O2����������¶��ֽ��ˡ�Ϊ����֤�����ڿ�����ȼ�յIJ������Ƿ���H2O2��ij����С��ͬѧ��Ƶ�ʵ��װ�ü�ͼ-1��

��3����ͬѧ�����ͼ-2�Ģ٣�����ѡȡ���ͼ��1�����е�װ�ã����е��� ����д��ţ���

��4������ͬѧ�����Ը��������Һ����H2O2�Ĵ��ڣ���ɸ÷�Ӧ�����ӷ���ʽ��
�� + �� Mn2+ + H2O
��ͬѧ���ҵļ��鷽����������ɣ���п����ϡ����ķ�Ӧ�в���������H2S�Ȼ�ԭ�����壬Ҳ��ʹ���Ը��������Һ��ɫ�������ͬѧ��ʵ�鷽������Ľ����飺 ��
��5����̼���ƣ�2Na2CO3?3H2O2���׳ƹ���˫��ˮ�����ֽ⣬��ֽⷴӦ�Ļ�ѧ����ʽ�ɱ�ʾΪ��2 (2Na2CO3?3H2O2) �� 4Na2CO3 + 6H2O + 3O2��
ȡһ�����Ĺ�̼�������ܱ�������ʹ����ȫ�ֽ⣬�����������12.0g����ȴ�����º������ò����м�ˮ���Ƴ�10.6% ��Na2CO3��Һ�����ˮ g��
��1��b��2�֣�
��2��21.25%����2�֣�д0.2125��ȫ�֣�
��3���ڣ�2�֣�
��4��2MnO4-+5H2O2+6H+��2Mn2++5O2��+8H2O��2�֣�������ȷдȫ1�֣���ƽ1�֣���
�Ƚ��Ƶõ�����ͨ��װ�м�ʯ�ҵĸ���ܣ�Ȼ���ȼ�������������֣���2�֣�
��5��433.5g��2�֣�
��2��21.25%����2�֣�д0.2125��ȫ�֣�
��3���ڣ�2�֣�
��4��2MnO4-+5H2O2+6H+��2Mn2++5O2��+8H2O��2�֣�������ȷдȫ1�֣���ƽ1�֣���
�Ƚ��Ƶõ�����ͨ��װ�м�ʯ�ҵĸ���ܣ�Ȼ���ȼ�������������֣���2�֣�
��5��433.5g��2�֣�
�����������1��˫��ˮ���Ȼ�ֽ⣬ֻ��ͨ����ѹ�����¶Ⱥ������Է���ֽ⣻
��2��˫��ˮ�к����������У�H2O2��H2O�������ʵ����ֱ�Ϊx��y������˫��ˮ����Ԫ�صĺ���Ϊ90%�����������Ĵ���Ϊ��ʾΪ��16/90%="18y+34x" ,(2x+3y)*16/(34x+18y)=90%,����ã�2x+y=1,��x=9-8/0.9�����34x/(34x+18y)=21.25%��
��3��ͼ��1�����е�װ������������װ�ã������������Ǣڣ�����ͨ��ʹ��Һ����Ӷ�ʹ��Ӧֹͣ������
��4������������ԭ��Ӧԭ������д��2MnO4-+5H2O2+6H+��2Mn2++5O2��+8H2O���൱����֤�������Ƿ���H2S���壬���ѻ������ͨ�뵽����ͭ��Һ����������ɫ����������H2S���塣Ҳ�����Ƚ��Ƶõ�����ͨ��װ�м�ʯ�ҵĸ���ܣ�Ȼ���ȼ��
��5�����ݷ���ʽ2 (2Na2CO3?3H2O2) �� 4Na2CO3 + 6H2O + 3O2������������������12.0gʱ������̼����12*4*106/(3*32)=53g������ˮ������Ϊ��(0.5*6/4)*18=13.5g��Ҫ���Ƴ�10.6% ��Na2CO3��Һ�����ˮ(53/0.106 )-53-13.5=433.5g��

��ϰ��ϵ�д�
�����Ŀ
 7N2��12H2O�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״���µ������ L��
7N2��12H2O�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״���µ������ L�� 2SO3(g) ��H��?196.6 kJ��mol�C1
2SO3(g) ��H��?196.6 kJ��mol�C1 ��
�� ��ͬ
��ͬ
 CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1
CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1