��Ŀ����
3���������ͭ������ؾ���{K2[Cu��C2O4��2]��2H2O}�Ʊ�������ͼ1��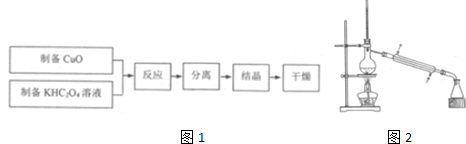
����֪��H2C2O4$\frac{\underline{\;\;��\;\;}}{\;}$CO��+CO2��+H20��
��1���Ʊ�CuO��CuSO4��Һ�е���NaOH��Һ��������У���ȴ��˫����ֽ���ˣ�ϴ�ӣ�
����˫����ֽ���˵�ԭ���Ƿ�ֹ��ֽ����
��������ˮϴ������ͭʱ�����֤������ͭ��ϴ�Ӹɾ�ȡ���һ��ϴ����Һ������BaCl2��Һ�����ް�ɫ������˵����ϴ�Ӹɾ���
��2��Ϊ�����CuO�������ʣ������CuO���ת�Ƶ��ȵ�KHC2O4��Һ��ֱ�ӽ�ϴ�Ӹɾ���CuO������ͬ��ֽһ��Ͷ�뵽KHC2O4��Һ�У�50��ˮԡ��������Ӧ��֣�������Ӧ�Ļ�ѧ����ʽΪ2KHC2O4+CuO$\frac{\underline{\;50��\;}}{\;}$K2[Cu��C2O4��2]+H2O���پ����ȹ��ˣ���ˮϴ�ӣ�����Һ����Ũ���õ��������ͭ������ؾ��壮
��3����ʵ����K2CO3��ĩ�������Һ��Ӧ�Ʊ�KHC2O4��������KOH��ĩ����K2CO3��ĩ������Ҫԭ����H2C2O4��KOH���������ķ��ȷ�Ӧ�����²�������ֽ⣮
��4���������ͭ������ؾ�����Ʊ�Ҳ������CuSO4�����K2C2O4��Һ��Ӧ�õ���������ͭ��Һ�л������ͭ�����ʵ�鲽��Ϊ�����������Ҵ�������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ����
�ټ��������Ҵ����ŵ��У�
a�����̼��ȵ�ʱ�䣬�����ܺģ�
b����������ͭ���ܽ�ȣ�����������ͭ�����������
��������Ũ���ij�ʼ�λ���������ͼ��ʾ��װ�ã���Ŀ���ǻ����Ҵ���
���� ��1���ٵ�����ֽ������������ֽ������
��CuO����ḽ����������ӣ����Ȼ�����Һ�������һ��ϴ��Һ���Ƿ�������������ж��Ƿ�ϴ�Ӹɾ���
��2������ʱ��ֽ��մ�д���������ͭ���壬����Ӧֱ�ӽ�ϴ�Ӹɾ�������ͭ������ͬ��ֽһ����뵽���������Һ�У���ַ�Ӧ��ȡ����ֽ��
CuO��KHC2O4��Һ��50��ˮԡ���������·�Ӧ����K2[Cu��C2O4��2]��H2O��
��3�������ֽ⣬���������ܽ���ȣ�����ᷴӦҲ���ȣ��ᵼ����Һ�¶ȹ��ߣ�����ֽ⣻
��4�����Ҵ����Խ�������ͭ���ܽ�ȣ�
��������Ҵ������л��գ�
��� �⣺��1���ٵ�����ֽ������������ֽ��������˫����ֽ�Ŀ���ԭ���Ƿ�ֹ��ֽ���𣬹ʴ�Ϊ����ֹ��ֽ����
��CuO����ḽ����������ӣ������Ƿ�ϴ�Ӹɾ��ľ��巽���ǣ�ȡ���һ��ϴ����Һ������BaCl2��Һ�����ް�ɫ������˵����ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ���һ��ϴ����Һ������BaCl2��Һ�����ް�ɫ������˵����ϴ�Ӹɾ���
��2������ʱ��ֽ��մ�д���������ͭ���壬����Ӧֱ�ӽ�ϴ�Ӹɾ�������ͭ������ͬ��ֽһ����뵽���������Һ�У���ַ�Ӧ��ȡ����ֽ��
���������֪��CuO��KHC2O4��Һ��50��ˮԡ���������·�Ӧ����K2[Cu��C2O4��2]��H2O����Ӧ�ķ���ʽΪ��2KHC2O4+CuO$\frac{\underline{\;50��\;}}{\;}$K2[Cu��C2O4��2]+H2O��
�ʴ�Ϊ��ֱ�ӽ�ϴ�Ӹɾ���CuO������ͬ��ֽһ��Ͷ�뵽KHC2O4��Һ�У�2KHC2O4+CuO$\frac{\underline{\;50��\;}}{\;}$K2[Cu��C2O4��2]+H2O��
��3����Ϊ�����ֽ⣬����������Ϊǿ���ܽ���ȣ�����ᷴӦҲ���ȣ��ᵼ����Һ�¶ȹ���ʹ����ֽ⣬���Բ���KOH��ĩ����K2CO3��ĩ��
�ʴ�Ϊ��H2C2O4��KOH���������ķ��ȷ�Ӧ�����²�������ֽ⣻
��4�����Ҵ����Խ�������ͭ���ܽ�ȣ�����������ͭ������������ʴ�Ϊ����������ͭ���ܽ�ȣ�����������ͭ�����������
��������Ҵ������л����Ҵ����ʴ�Ϊ�������Ҵ���
���� ������Ҫ��ѧʵ���Ʊ��ۺ�Ӧ�ã�����ʵ��������������������ǽ���ؼ����Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

| A�� | �������Ƶ���ʽ��Na${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$Na | |
| B�� | ������35��������45����ԭ�ӣ�${\;}_{35}^{80}$Br | |
| C�� | �����ӽṹʾ��ͼ��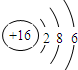 | |
| D�� | ��ϩ�Ľṹ��ʽ��C2H4 |
| A�� | CuO��CuCl2 | B�� | K2SO4��KCl | C�� | CaCO3��CaCl2 | D�� | Mg��OH��2��MgCl2 |
| A�� | 1��1 | B�� | 2��3 | C�� | 3��2 | D�� | 1��3 |
| A�� | H2O��D2O | B�� | O2��O3 | ||
| C�� | CH3CH2OH��CH3-O-CH3 | D�� | 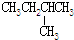 �� ��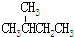 |
| A�� | 18 gˮ���������ӵ����ʵ���Ϊ10 mol | |
| B�� | N2��CO��Ħ��������ȣ�����28 | |
| C�� | 1 mol CO2������Ϊ44 g•mol-1 | |
| D�� | ����������Ħ��������Ϊ98 g |
| A�� | ��ϵͳ��������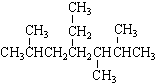 ������Ϊ2��5��6-����-4-�һ����� ������Ϊ2��5��6-����-4-�һ����� | |
| B�� | ��ѹ�£������顢�����顢������ķе��������� | |
| C�� | �����ࡱ���ʾ��������Ե���ζ | |
| D�� | 1mol��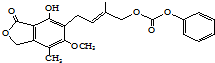 ������뺬5mol NaOH��ˮ��Һ��ȫ��Ӧ ������뺬5mol NaOH��ˮ��Һ��ȫ��Ӧ |
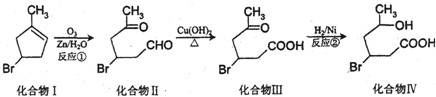
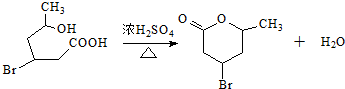 ��
��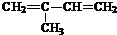 ��2-��-1��3-����ϩ���������Ƣٵķ�Ӧ���õ��л���VI��VII���ṹ��ʽ�ֱ���Ϊ
��2-��-1��3-����ϩ���������Ƣٵķ�Ӧ���õ��л���VI��VII���ṹ��ʽ�ֱ���Ϊ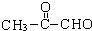 ��HCHO��
��HCHO��