ћвƒњƒЏ»Ё
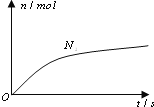
°Њћвƒњ°њ—–Њњµзљв÷ ‘ЏЋЃ»№“Ї÷–µƒ∆љЇвƒ№ЅЋљвЋьµƒіж‘Џ–ќ љ£ђ”–÷Ў“™µƒ µЉ “в“е°£

£®1£©≥£ќ¬ѕ¬£ђѕт100mL0.01mol°§L-1HAµƒ»№“Ї÷–÷рµќЉ”»л0.02mol°§L-1MOH»№“Ї£ђЋщµ√»№“ЇµƒpHЋжMOH»№“Їµƒћеїэ±дїѓ»зЌЉЋщ Њ£®»№“Їћеїэ±дїѓЇц¬‘≤їЉ∆£©°£
Ґў≥£ќ¬ѕ¬£ђ0.01mol°§L-1HA»№“Ї÷–”…ЋЃµзјл≥цµƒc£®H+£©=_____mol°§L-1°£
ҐЏ≥£ќ¬ѕ¬“їґ®≈®ґ»µƒMAѕ°»№“ЇµƒpH=a£ђ‘тa____7£®ћо°∞£Њ°±°Ґ°∞£Љ°±їт°∞=°±£©£ђ”√јл„”Јљ≥ћ љ±н Њ∆д‘≠“тќ™_____°£
ҐџXµг ±£ђ»№“Ї÷–c£®H+£©°Ґc£®M+£©°Ґc£®A-£©”…іуµљ–°µƒЋ≥–т «_____°£
Ґ№Kµг ±£ђ»№“Ї÷–c£®H+£©+ c£®M+£©- c£®OH-£©=____molL-1°£
£®2£©25 °ж ±£ђ“—÷™Ka£®CH3COOH£©£љ1.75°Ѕ10£≠5£ђKb£®NH3°§H2O£©£љ1.76°Ѕ10£≠5°£»°≈®ґ»Њщќ™0.100 0 mol°§L£≠1µƒі„Ћб»№“ЇЇЌ∞±ЋЃ»№“ЇЄч20.00 mL”Џ„ґ–ќ∆њ÷–£ђЈ÷±р”√0.1000 mol°§L£≠1NaOH»№“Ї°Ґ0.1000 mol°§L£≠1—ќЋбљш––µќґ®£ђµќґ®єэ≥ћ÷–pHЋжµќЉ”»№“Їћеїэ±дїѓєЎѕµ»зЌЉЋщ Њ°£
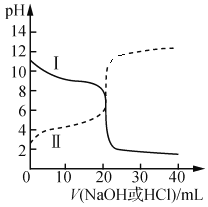
Ґўі”«ъѕяIњ…÷™£ђЄ√µќґ®≤ў„ч ±£ђ”¶—°______„чќ™÷Є ЊЉЅ°£
ҐЏ‘Џ«ъѕяҐт£ђµ±µќЉ”»№“Їµљ10.00 mL ±£Ї
c£®CH3COO£≠£©£Ђc£®OH£≠£© ___ c£®H£Ђ£©£Ђc£®CH3COOH£© £®ћо°∞£Њ°±°Ґ°∞£Љ°±їт°∞=°±£©
°Њір∞Є°њ10-12 < M++ H2OMOH+H+ c(A-)> c(H+)> c(M+) 0.0005 Љ„їщ≥» >
°Њљвќц°њ
(1)Ґў”…pH÷™£ђHAќ™«њЋб£ђ»№“Ї÷–”…ЋЃµзјл≥цµƒc(H+)µ»”Џ»№“Ї÷–µƒOH-≈®ґ»°£
ҐЏґ‘”Џ«њЋб»хЉо—ќ£ђљр фјл„”ЈҐ…ъЋЃљв£ђ»№“Їѕ‘Ћб–‘°£
ҐџXµг ±£ђі”»№“Ї÷–µƒ»№÷ Љ∞µзјлƒ№Ѕ¶љш––Ј÷ќц£ђі”ґшµ√≥ц»№“Ї÷–c(H+)°Ґc(M+)°Ґc(A-)”…іуµљ–°µƒЋ≥–т°£
Ґ№і”µзЇ… ЎЇгљш––Ј÷ќц£ђ«у≥цc(A-)°£
(2) Ґў“тќ™µќґ®÷’µг ±£ђ»№“Ї≥ Ћб–‘£ђ”¶—°±д…ЂЈґќІµг‘ЏЋб–‘«ш”тµƒ÷Є ЊЉЅ°£
ҐЏјы”√Є√µг»№“Їµƒ„й≥…Љ∞µзјл”лЋЃљвµƒ÷чіќ£ђ»Јґ®ќҐЅ£≈®ґ»µƒѕаґ‘іу–°°£
£®1£©і”ЌЉ÷–њ…“‘њі≥ц£ђ0.01mol°§L-1HAµƒpH=2£ђЋµ√ч∆дќ™«њЋб°£
Ґў≥£ќ¬ѕ¬£ђ0.01mol°§L-1HA»№“Ї÷–”…ЋЃµзјл≥цµƒc(H+)= c(OH-)=![]() mol°§L-1£ї
mol°§L-1£ї
ір∞Є£Ї10-12°£
ҐЏµ±pH=7 ±£ђV(MOH)=51mL£ђn(HA)=0.001mol£ђn(MOH)=0.00102mol£ђMOHєэЅњ£ђі”ґшЋµ√чMAќ™«њЋб»хЉо—ќ£ђpH=a£ђ‘тa<7£ї
јл„”Јљ≥ћ љ±н Њ∆д‘≠“тќ™M++ H2OMOH+H+£ї
ір∞Є£Ї<£їM++ H2OMOH+H+°£
ҐџXµг ±£ђn(HA)=0.001mol£ђn(MOH)=0.0005mol£ђЈі”¶…ъ≥…n(MA)=0.0005mol£ђ £”аn(HA)=0.0005mol£ђ»№“Ї÷–c(H+)°Ґc(M+)°Ґc(A-)”…іуµљ–°µƒЋ≥–т «c(A-)> c(H+)> c(M+)£ї
ір∞Є£Їc(A-)> c(H+)> c(M+)°£
Ґ№Kµг ±£ђn(HA)=0.001mol£ђn(MOH)=0.002mol£ђЈі”¶…ъ≥…n(MA)=0.001mol£ђ £”аn(HA)=0.001mol£ђ»№“Ї÷–c(H+)+ c(M+)- c(OH-)= c(A-)=0.0005molL-1°£
ір∞Є£Ї0.0005°£
£®2£©і”і„ЋбЇЌ∞±ЋЃµƒЋбЉо–‘≈–ґѕ£ђ«ъѕяIќ™∞±ЋЃ£ђ«ъѕяҐтќ™і„Ћб°£
Ґўі”«ъѕяIњ…÷™£ђЄ√µќґ®≤ў„ч ±£ђµќґ®÷’µг‘ЏЋб–‘«ш”тƒЏ£ђ”¶—°Љ„їщ≥»„чќ™÷Є ЊЉЅ£ї
ір∞Є£ЇЉ„їщ≥»°£
ҐЏ‘Џ«ъѕяҐт£ђµ±µќЉ”»№“Їµљ10.00 mL ±£ђn(CH3COOH)=0.002mol£ђn(NaOH)=0.001mol£ђ
Јі”¶Їу»№“Ї÷–£ђn(CH3COOH)=0.001mol£ђn(CH3COONa)=0.001mol£ђ“‘і„Ћбµƒµзјлќ™÷ч£ђ
c(CH3COO£≠)£Ђc(OH£≠) >c(H£Ђ)£Ђc(CH3COOH) £ї
ір∞Є£Ї>°£

°Њћвƒњ°њќ™ЅЋЄь…оњћµЎ»ѕ ґ¬±ЋЎµƒ–‘÷ £ђƒ≥їѓ—І–°„йґ‘¬±ЋЎЉ∞∆дїѓЇѕќпµƒ÷∆±ЄЇЌ–‘÷ љш––»зѕ¬ћљЊњ µ—й£ђЄщЊЁ µ—йїЎірќ ћв°£
[ µ—й“ї]¬»∆шµƒ÷∆±Є
£®1£©Є√–°„йƒв”√ЌЉЉ„ µ—й„∞÷√јі÷∆±ЄіњЊї°ҐЄ…‘пµƒ¬»∆ш£ђ≤ҐЌк≥…”лљр фћъµƒЈі”¶(Љ–≥÷“«∆ч¬‘»•)°£√њЄц–йѕяњт±н Њ“їЄцµ•‘™„∞÷√£ђ«л”√ќƒ„÷√и цљЂѕ¬Ѕ–„∞÷√µƒінќу÷Ѓі¶Єƒ’э£Ї___°£
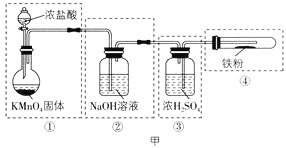
[ µ—йґю]ћљЊњ¬»їѓ—«ћъ”л—х∆шЈі”¶µƒ≤ъќп
“—÷™¬»їѓ—«ћъµƒ»џµгќ™674°ж£ђЈ–µгќ™1023°ж£ї»э¬»їѓћъ‘Џ100°ж„у”“ ±…эї™£ђЉЂ“„ЋЃљв°£‘Џ500°жћхЉюѕ¬¬»їѓ—«ћъ”л—х∆шњ…ƒ№ЈҐ…ъѕ¬Ѕ–Јі”¶£Ї12FeCl2+3O2![]() 2Fe2O3+8FeCl3°Ґ4FeCl2+3O2
2Fe2O3+8FeCl3°Ґ4FeCl2+3O2![]() 2Fe2O3 +4Cl2£ђЄ√їѓ—І–°„й—°”√ЌЉ““≤њЈ÷„∞÷√(„∞÷√њ…“‘÷ЎЄі—°”√)љш––¬»їѓ—«ћъ”л—х∆шЈі”¶≤ъќпµƒћљЊњ°£
2Fe2O3 +4Cl2£ђЄ√їѓ—І–°„й—°”√ЌЉ““≤њЈ÷„∞÷√(„∞÷√њ…“‘÷ЎЄі—°”√)љш––¬»їѓ—«ћъ”л—х∆шЈі”¶≤ъќпµƒћљЊњ°£
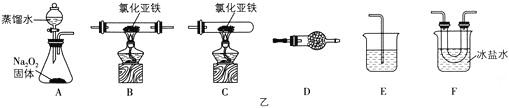
£®2£© µ—й„∞÷√µƒЇѕјнЅђљ”Ћ≥–тќ™A°ъ___°ъE°£
£®3£©Љт цљЂ„∞÷√F÷–µƒєћће≈д≥…»№“Їµƒ≤ў„чЈљЈ®£Ї___°£
[ µ—й»э]¬±ЋЎїѓЇѕќп÷ЃЉдЈі”¶µƒ µ—йћхЉюњЎ÷∆ћљЊњ
£®4£©‘Џ≤їЌђ µ—йћхЉюѕ¬KClO3њ…љЂKI—хїѓќ™I2їтKIO3°£Є√–°„йЌђ—І…иЉ∆µƒ“ї„й µ—йµƒ эЊЁЉ«¬Љ»зѕ¬±н( µ—йњЎ÷∆‘Џ “ќ¬ѕ¬љш––)£Ї
‘є№±кЇ≈ | 1 | 2 | 3 | 4 |
0.20molL-1KI»№“Ї/mL | 1.0 | 1.0 | 1.0 | 1.0 |
KClO3(s)/g | 0.10 | 0.10 | 0.10 | 0.10 |
6.0molL-1H2SO4»№“Ї/mL | 0 | 3.0 | 6.0 | 9.0 |
’фЅуЋЃ/mL | 9.0 | 6.0 | 3.0 | 0 |
µ—йѕ÷ѕу |
ҐўЄ√„й µ—йµƒƒњµƒ «___°£
ҐЏ2Ї≈ ‘є№Јі”¶Ќк»ЂЇу£ђ»°…ўЅњ ‘є№÷–µƒ»№“Ї£ђµќЉ”µнЈџ»№“ЇЇуѕ‘јґ…Ђ£ђЉў…иїє‘≠≤ъќп÷ї”–KCl£ђ–і≥цЈі”¶µƒјл„”Јљ≥ћ љ£Ї___°£