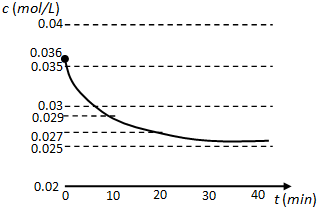
��Ŀ����
2�������й���Һ�����������ӣ������ж�����ȷ���ǣ�������| A�� | ����������ʹ����ʯ��ˮ����ǵ��������ɣ���ԭ��Һ��һ���д�����CO$\stackrel{2-}{3}$���� | |
| B�� | ��ij��Һ����ɫ��Ӧʵ�����Ϊ��ɫ�������Һ��һ������Ԫ�أ������м�Ԫ�� | |
| C�� | �������ữ���ٵμ�KSCN��Һ���к�ɫ�������ɣ���ԭ��Һ��һ����Fe3+���� | |
| D�� | �ֱ���Mg2+��Cu2+��Fe2+��Na+��������������Һ��ֻ��NaOH��Һ�Dz���һ�μ��� |
���� A���ܹ�ʹ����ʯ��ˮ����ǵ������п���Ϊ�����������壻
B����������ɫ��ӦΪ��ɫ���۲��������ɫ��Ӧ������ɫ��Ҫ����ɫ�ܲ�����
C��������������ԣ��ܹ����������������������ӣ�
D��Mg2+��Cu2+��Fe2+���������Ʒ�Ӧ�ֱ����ɰ�ɫ��������ɫ��������ɫ��״����������Ѹ�ٱ�ɻ���ɫ��
��� �⣺A���ܹ�ʹ����ʯ��ˮ����ǵ������ж�����̼�Ͷ�����������ԭ��Һ�п��ܴ���̼��������ӡ�����������ӡ�������������ӣ�����ԭ��Һ�в�һ������̼������ӣ���A����
B����Ԫ����ɫ��Ӧ�Ļ���Ϊ��ɫ������ֱ�ӹ۲쵽������Ԫ����ɫ��ӦΪ��ɫ�����ܱ��ƹ���ס���۲�ʱ��Ҫ����ɫ���ܲ����۲죬������Һ�п��ܴ��ڼ����ӣ���B��ȷ��
C�����������ữ������Һ�����������ӣ��ܹ������������������ӣ����Եμ�KSCN��Һ����Ѫ��ɫ�������ɣ�ԭ��Һ�п��ܴ����������ӣ���һ�����������ӣ����������ӷ���Ӧ��������Һ�м������軯����Һ������Һ��ɺ�ɫ��֤���������Ӵ��ڣ���C����
D������Mg2+��Cu2+��Fe2+��Na+����������Һ�зֱ�����������ƣ�þ�����������������ɰ�ɫ������ͭ��������������������ɫ���������������ܹ��������������ɰ�ɫ��״������Ѹ�ٱ�ɻ���ɫ��û�����������Ϊ�����ӣ����Կ���ʹ���������Ƽ����������ӣ���D����
��ѡB��
���� ���⿼���˳������ӵļ��鷽������Ŀ�ѶȲ�����������������ѧ������˼ά�����ͷ�ɢ˼ά���������ѧ����Ӧ��������ѧϰЧ�ʣ�����������Ҫע����ǽ������Ӽ���ʱ��Ҫ�������ӵ��������ʺ�������Ӧ�����жϣ�

 ��У����ϵ�д�
��У����ϵ�д�| ������ | K+ Na+ Cu+ Al+ |
| ������ | SO${\;}_{4}^{2-}$ HCO${\;}_{3}^{-}$ NO${\;}_{3}^{-}$ OH- |
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��cΪ��ɫ������ɫ�겣������
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�ж��ܲ�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɣ�
��������ʵ����գ�
��1��д��B��D�Ļ�ѧʽ��BKNO3��DCuSO4��
��2������1 mol A����Һ�뺬l molE����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ļ�ѧʽΪNa2CO3��
��3����A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ2HCO3-+Ca2++2OH-=CaCO3��+CO32-+H2O��
��4��C��������ˮ���������ӷ���ʽ���ʵ�����˵���侻ˮԭ��Al3++3H2O?Al��OH��3�����壩+3H+ˮ�����ɵ�������������������ˮ�е������ᄏˮ
��5����������lmol��C��Һ����μ���Ba��OH��2��Һ�����ɳ����������Ϊ466g��
| A�� | AlCl3 | B�� | KHCO3 | C�� | Fe2��SO4��3 | D�� | NH4HCO3 |
���飺��ˮ ������������Һ �����Ȼ�̼ ��ʳ��
���飺A���ᾧ B������ C����ȡ D����Һ E���ܽ�
| ��� | ����� | �Լ���� | ������� |
| ��1�� | ����غ��Ȼ��ƵĹ������� | ||
| ��2�� | �Ȼ��غ͵�Ļ����Һ | ||
| ��3�� | ���ͺ�ˮ�Ļ���� | ||
| ��4�� | �Ҷ������е�198�棩�ͱ��������е�290�棩�Ļ���� | �� |
| A�� | ��һ�������¿ɽ�ʯīת��Ϊ���ʯ | |
| B�� | ��������������������ᷴӦ�������ų����� | |
| C�� | ��ȡþ��ʱ���ɽ�þ�����ڵ�������ȴ | |
| D�� | SO2��ʹƷ����Һ��ɫ��������ʹ��ɫʯ����Һ��ɫ |
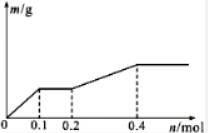 ��Pt�缫��⺬��Ag+��Cu2+��X3+��0.1mol����Һ�����������������ʵ�����m��g�����·��ͨ�����ӵ����ʵ���n��mol���Ĺ�ϵ��ͼ��ʾ��������������ǿ�����ж���ȷ���ǣ�������
��Pt�缫��⺬��Ag+��Cu2+��X3+��0.1mol����Һ�����������������ʵ�����m��g�����·��ͨ�����ӵ����ʵ���n��mol���Ĺ�ϵ��ͼ��ʾ��������������ǿ�����ж���ȷ���ǣ�������| A�� | Ag+��X3+��Cu2+��H+��X2+ | B�� | Ag+��Cu2+��X3+��H+��X2+ | ||
| C�� | Cu2+��X3+��Ag+��X2+��H+ | D�� | Cu2+��Ag+��X3+��H+��X2+ |
| A�� | ��¯�г�����ˮ���ݿ�����ϡ�����ܽ�ȥ�� | |
| B�� | ����FeCl3������Һ�Ʊ�Fe��OH��3�ٽ���Fe3+ˮ�� | |
| C�� | ��ˮ�м���������¶���ʹˮ�����ӻ���С��ˮ�ĵ���ƽ�ⶼ�����ƶ� | |
| D�� | ��Ӧ2A��g��+B��g���T3C��s��+D��g����һ�����������Է����У�˵���÷�Ӧ�ġ�H��0 |
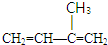 ��
��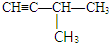 ��
��