��Ŀ����
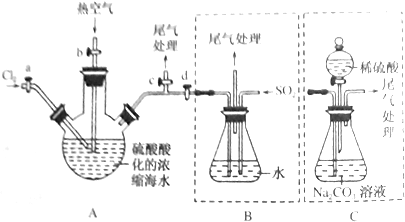
10��Ϊ�����±��еĻ����ڼ�����ѡ�����õ��Լ���������ѡ�����õ���Ҫ����������������������Ӧ���У����������Լ�������д��/�������飺��ˮ ������������Һ �����Ȼ�̼ ��ʳ��
���飺A���ᾧ B������ C����ȡ D����Һ E���ܽ�
| ��� | ����� | �Լ���� | ������� |
| ��1�� | ����غ��Ȼ��ƵĹ������� | ||
| ��2�� | �Ȼ��غ͵�Ļ����Һ | ||
| ��3�� | ���ͺ�ˮ�Ļ���� | ||
| ��4�� | �Ҷ������е�198�棩�ͱ��������е�290�棩�Ļ���� | �� |
���� ��1������غ��Ȼ��Ƶ��ܽ�����¶ȵı仯��ͬ��
��2�������������Ȼ�̼��
��3�����ͺ�ˮ�ֲܷ㣻
��4���Ҷ����ͱ������ķе㲻ͬ��
��� �⣺��1������غ��Ȼ��Ƶ��ܽ�����¶ȵı仯��ͬ���ɼ���ˮ�ܽ⣬Ȼ���ýᾧ�ķ������룻
��2�������������Ȼ�̼��������ȡ����Һ���룻
��3�����ͺ�ˮ�ֲܷ㣬���÷�Һ���룻
��4���Ҷ����ͱ������ķе㲻ͬ������������룻
�ʴ�Ϊ��
| ��� | ����� | �Լ���� | ������� |
| ��1�� | ����غ��Ȼ��ƵĹ������� | �� | E��A |
| ��2�� | �Ȼ��غ͵�Ļ����Һ | �� | C��D |
| ��3�� | ���ͺ�ˮ�Ļ���� | �� | D |
| ��4�� | �Ҷ������е�198�棩�ͱ��������е�290�棩�Ļ���� | B |
���� ���⿼�����ʵķ��롢�ᴿ֪ʶ����Ŀ�ѶȲ���ע��������ʵ����ʵ���ͬѡ��ʵ�鷽����ѧϰ��Ҫע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
5����ѧ����������������������أ������й�˵������ȷ���ǣ�������
| A�� | Ѥ���ͷ��̻��������˺��أ��ƣ��ƣ�ͭ�Ƚ���Ԫ�ػ����� | |
| B�� | ̼�����ƿ������Ʊ��������θ������ҩ�ʳƷ���ͼ� | |
| C�� | ҽ�þƾ�ʹ�õ�����ֲ��;������Ƴɣ�Ũ��ͨ��Ϊ75% | |
| D�� | ����ˮ���������Խ������ˮ��ӦΣ��������ˮ�м��뾻ˮ����������ʹ��ˮ���� |
2�������й���Һ�����������ӣ������ж�����ȷ���ǣ�������
| A�� | ����������ʹ����ʯ��ˮ����ǵ��������ɣ���ԭ��Һ��һ���д�����CO$\stackrel{2-}{3}$���� | |
| B�� | ��ij��Һ����ɫ��Ӧʵ�����Ϊ��ɫ�������Һ��һ������Ԫ�أ������м�Ԫ�� | |
| C�� | �������ữ���ٵμ�KSCN��Һ���к�ɫ�������ɣ���ԭ��Һ��һ����Fe3+���� | |
| D�� | �ֱ���Mg2+��Cu2+��Fe2+��Na+��������������Һ��ֻ��NaOH��Һ�Dz���һ�μ��� |
20������ԭ�ӵĹ����ʾʽ��ȷ���ǣ�������
| A�� |  | B�� |  | C�� |  | D�� |  |
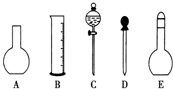 ʵ������Ҫ0.1mol•L-1 NaOH��Һ450mL��0.5mol•L-1������Һ500mL��������������Һ����������ش��������⣺
ʵ������Ҫ0.1mol•L-1 NaOH��Һ450mL��0.5mol•L-1������Һ500mL��������������Һ����������ش��������⣺