��Ŀ����
������Һ�д��ڵ���ƽ��CH3COOH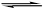 H����CH3COO����������������ȷ����
H����CH3COO����������������ȷ����
| A��������Һ������Ũ�ȵĹ�ϵ���㣺c(H��)��c(OH��)��c(CH3COO��) |
| B��0.10mol/L��CH3COOH��Һ�м�ˮϡ�ͣ���Һ��c(OH��)��С |
| C��CH3COOH��Һ�м�������CH3COONa���壬ƽ�������ƶ� |
| D��������pH��2��CH3COOH��Һ��pH��12��NaOH��Һ�������Ϻ���Һ��pH��7 |
B
�������������A�����ݵ���غ㣬H+�������������������OH?��CH3COO?�����������������ȷ��B��CH3COOH��Һ�м�ˮϡ�ͣ�H+Ũ�ȼ�С����ΪH+Ũ����OH?Ũ�ȳ˻�Ϊ������OH?Ũ��������C������������CH3COONa��CH3COO?Ũ������ƽ�������ƶ�����ȷ��D��CH3COOHΪ���ᣬpH=2��CH3COOHŨ��ԶԶ����0.01mol?L?1�������������Һ��pH��7����ȷ��
���㣺���⿼��������ʵĵ��롢����غ㡢����к�Ӧ��

��֪���ᡢ�������������Һ�д�������ƽ�⣺
CH3COOH+H2O 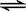 CH3COO��+H3O+ K1=1��75��10-5
CH3COO��+H3O+ K1=1��75��10-5
CH3COO��+H2O 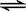 CH3COOH+OH�� K2=5��71��10-10
CH3COOH+OH�� K2=5��71��10-10
����50 mL 0��2 mol��L-1������50 mL 0��2 mol��L-1�����ƻ���Ƶ���Һ�ף�����������ȷ����
| A������Һ��pH ��7 |
| B���Լ����ȣ�K1��K2ͬʱ���� |
| C�����ڼ��м����������NaOH��Һ����Һ��pH�������� |
| D�����ڼ��м���5 mL 0��1 mol��L-1�����ᣬ����Һ�д����K1���� |
�����йص������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ����( )
| A��pH=3��һԪ���pH=11��һԪǿ��������Ϻ����Һ�У�c(OH��)=c(H+) |
| B����0.1 mol��L��1Na2CO3��Һ�У�c(OH��)��c(H��)��c(HCO3��)��2c(H2CO3) |
| C����֪������(HN3)������������������NaN3ˮ��Һ������Ũ�ȴ�С˳��Ϊ��c(Na+)��c(OH¯)��c(N3¯)��c(H��) |
| D��0.2 mol��L��1NaHCO3��Һ�м�������0.1 mol��L��1NaOH��Һ��c(CO32��)��c(HCO3��)��c(OH��)��c(H��) |
��ˮ�ĵ���ƽ�ⲻ����Ӱ���������
| A��HCl | B��CH3COO�� | C��Cl�� | D��Fe3+ |
ij�¶�ʱ��BaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵����ȷ���ǣ���ʾ��BaSO4(s) Ba2+(aq)+SO42-(aq)��ƽ�ⳣ��Ksp = c(Ba2+)��c(SO42-)����Ϊ�ܶȻ���������
Ba2+(aq)+SO42-(aq)��ƽ�ⳣ��Ksp = c(Ba2+)��c(SO42-)����Ϊ�ܶȻ���������
| A������Na2SO4����ʹ��Һ��a��䵽b�� |
| B��d����BaSO4�������� |
| C��ͨ����������ʹ��Һ��d��䵽c�� |
| D��a���Ӧ��Ksp����c���Ӧ��Ksp |
�������ʼ���ˮ�У���ˮ�ĵ����ܲ����ٽ����õ���
| A��NH4Cl | B��NaOH | C��NaCl | D��H2SO4 |
��֪25��ʱ��Ka(HF)��3.6��10-4��Ksp(CaF2)��1.46��10-10������1L0.2mol��L-1HF��Һ�м���1L0.2mol��L-1CaCl2��Һ��������˵���У���ȷ����
| A��25��ʱ��0.lmol��L-1��Һ��pH��l |
| B��Ksp(CaF2)���¶Ⱥ�Ũ�ȵı仯���仯 |
| C������ϵ��û�г������� |
| D������ϵ��HF��CaC12��Ӧ�������� |
������Һ������˵����ȷ����
| A��c(H��):c(OH��)��1:10-2����Һ��K����Ba2����ClO����CO32��һ���ܴ������� |
| B��ˮ���������c(H��)��10-13mol/L����Һ��K����Cl����NO3����I��һ���ܴ������� |
C����0.1mol/LCH3COOH��Һ��ͨ������HCl������ĵ���ƽ�����淴Ӧ�����ƶ�������Һ�� ���� ���� |
| D�������ʵ���Ũ�ȵ�������Һ����H2CO3��Na2CO3��NaHCO3��(NH4)2CO3��c(CO32��)�Ĵ�С��ϵΪ����>��>��>�� |