��Ŀ����
����Ŀ������ѧ��ѡ�ޣ����ʽṹ�����ʡ�
�����ѣ�22Ti���ųƺ��ղ��ϡ��ش��������⣺
(1)��Ԫ�ػ�̬ԭ��δ�ɶԵ�����Ϊ________����������ߵĵ���ռ�ݵ��ܼ�����Ϊ___________�����ܼ������ܲ���е�ԭ�ӹ����Ϊ_____________��
(2)[Ti(OH)2(H2O)4]2+�еĻ�ѧ����_____________��
a.�Ҽ� b.�м� c.���Ӽ� d.��λ��
(3)����TiO2��һ��Ӧ�ù㷺�Ĵ���������TiO2����һ��ʵ����ͼ��ʾ��

������ķ����в�ȡsp2�ӻ���ʽ��̼ԭ�Ӹ���Ϊ__________�����������в�ȡsp3�ӻ���ԭ�ӵĵ�һ��������С�����˳��Ϊ____________��
(4)��ҵ���ƽ����Ѳ��ý�����ԭ���Ȼ��ѡ��Ƚ�TiO2������Ȼ�Ľ��ʯ��������̿�ۻ�ϼ�����1000~1100K�������Ȼ�����������TiCl4.д������TiCl4�Ļ�ѧ��Ӧ����ʽ��_______________��
(5)��һ�ֵ����Ѿ���ľ�����ͼ��ʾ���þ���Ļ�ѧʽΪ____________���þ�����Tiԭ����Χ������������ȵ� Nԭ�ӵĸ���Ϊ___________����֪������ܶ�Ϊ��g��cm-3�������ӵ�����ΪNA�����߳�Ϊ________cm���ú��ѡ�NA��ʽ�ӱ�ʾ����

���𰸡�23d9ad7C<O<NTiO2+ 2C+2C12 ![]() TiCCl4+2COTiN4
TiCCl4+2COTiN4![]()
��������(1)TiԪ�ؼ۵����Ų�ʽΪ3d24s2����̬ԭ�Ӽ۵����Ų�ͼΪ![]() ���ɼ���Ԫ�ػ�̬ԭ��δ�ɶԵ�����Ϊ2����������ߵĵ���ռ�ݵ��ܼ�����Ϊ3d�����ܼ������ܲ�ΪM�ܲ㣬��3s��3p��3d���ֱ���ԭ�ӹ����ĿΪ1��3��5�����е�ԭ�ӹ����Ϊ9��
���ɼ���Ԫ�ػ�̬ԭ��δ�ɶԵ�����Ϊ2����������ߵĵ���ռ�ݵ��ܼ�����Ϊ3d�����ܼ������ܲ�ΪM�ܲ㣬��3s��3p��3d���ֱ���ԭ�ӹ����ĿΪ1��3��5�����е�ԭ�ӹ����Ϊ9��
(2)[Ti(OH)2(H2O)4]2+�еĻ�ѧ��ֻ�ЦҼ�����λ��������Ϊad��
��3����ȡsp2�ӻ���̼ԭ�Ӽ۲���Ӷ�����3���÷�����̼ԭ�Ӽ۲���Ӷ���Ϊ3���У������ϵ�̼ԭ�ӡ������ʻ���̼ԭ�ӣ�����һ����7������ȡsp3�ӻ���ԭ�Ӽ۲���Ӷ�����4���۲���ӶԸ�����4��ԭ���У����Ӽ����ǻ���̼ԭ�ӡ���ԭ�Ӻ͵�ԭ�ӣ�ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����VA��Ԫ�ش�������Ԫ�أ�����������Ԫ�ص�һ�����ܴ�С����˳���ǣ�C��O��N��
(4)TiO2������̿�ۻ�ϼ�����1000~1100K������TiCl4�Ļ�ѧ��Ӧ����ʽΪ2C+2C12 ![]() TiCCl4+2CO��
TiCCl4+2CO��
(5)�۲쾧��Nλ��������Ķ��������λ�ã�N��=8��![]() +6��
+6��![]() =4��Tiλ�ھ����ڣ���4������ѧʽ��дΪTiN���ɾ���ͼ��֪��Ti������N���ĸ�������������壻��������Ϊ4��
=4��Tiλ�ھ����ڣ���4������ѧʽ��дΪTiN���ɾ���ͼ��֪��Ti������N���ĸ�������������壻��������Ϊ4��![]() g��������ܶ�Ϊ�� gcm-3�����߳�Ϊ
g��������ܶ�Ϊ�� gcm-3�����߳�Ϊ![]() =
=![]() cm��
cm��

����Ŀ���ұ���Ԫ�����ڱ���һ���֣�����A��B��D��E��G��JΪ������Ԫ�أ�GԪ�صĺ˵����ΪBԪ�ص�2������ش��������⣺
A | B | ||
D | E | G | J |
L | M | Q |
��1��J������������Ӧˮ����Ļ�ѧʽ��___________________��
��2��G2J2�������ĵ�����������������ʽΪ_______________________��д��һ���ܱ�ʾԪ��G��J�ǽ�����ǿ����ϵ�Ļ�ѧ����ʽ____________________________��
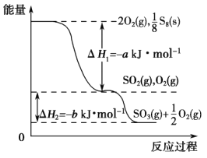
��3��G�����ڿ�����ȼ������һ����ɫ�д̼�����ζ�����壬����ɫ�д̼�����ζ�������뺬1molJ��һ�ֺ�����(�����ij�γ�����ʵ������ȡ����)����Һ�ڡ��������·�Ӧ��������һ��ǿ���һ��������Ҹ÷�Ӧ����NA������ת�ƣ���÷�Ӧ�Ļ�ѧ����ʽ��________________________��
��4��A������⻯����___________������ԡ��Ǽ��ԡ������ӣ�ʵ�����Ʊ������ʵĻ�ѧ����ʽΪ__________________________________________��
��5������Ԫ�������ɣ������Ʋ��ϱ��г�����Ԫ�صĵ��ʾ��а뵼�����Ե���__________����Ԫ�ط��ţ���
����Ŀ��ijԭ����ܷ�Ӧ�����ӷ���ʽ��Fe+2Fe3+=3Fe2+ �� ��ԭ��ص������ȷ���ǣ� ��
Zn | A | B | C | D |
���� | Fe | C��ʯī�� | Ag | Zn |
���� | Cu | Fe | Fe | Fe |
�������Һ | FeCl3 | Fe2 ��SO4��3 | H2SO4 | FeCl3 |
A.A
B.B
C.C
D.D