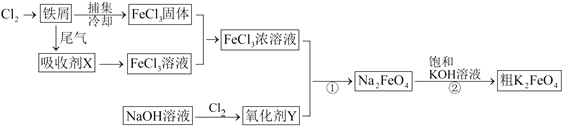
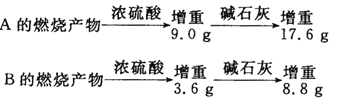
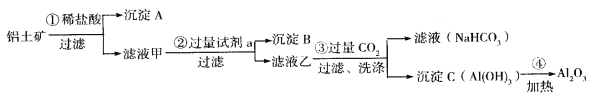
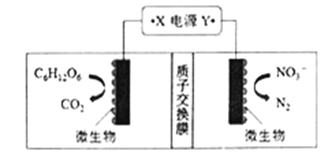
��Ŀ����
����Ŀ����1������ˮ����ֳЭ�������һ�����õ绯ѧԭ�����������ˮ�ʵķ�������װ����ͼ��ʾ����д�������ĵ缫��Ӧʽ _________________________��
��2���״�������ˮú���ϳɣ�CO(g)+2H2(g) ![]() CH3OH(g) ��H<0. һ�������£���1molCO��2molH2ͨ���ܱ������н��з�Ӧ�����ı��¶Ȼ�ѹǿʱ��ƽ���CH3OH��������� ��CH3OH���仯������ͼ��ʾ������˵����ȷ����___________��
CH3OH(g) ��H<0. һ�������£���1molCO��2molH2ͨ���ܱ������н��з�Ӧ�����ı��¶Ȼ�ѹǿʱ��ƽ���CH3OH��������� ��CH3OH���仯������ͼ��ʾ������˵����ȷ����___________��
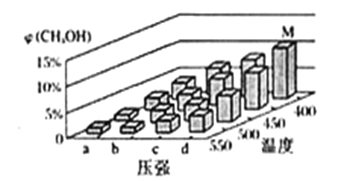
A.״̬M��ƽ��ʱ��COת����Ϊ10%
B.ͼ��ѹǿ�Ĵ�С��ϵ��a<b<c<d
C.���º�ѹʱ����ԭƽ����ϵ���ٳ��������״�������ƽ�����ϵ�м״��������������
D.����ϵ�� n(CO)/n(H2)��ֵ����ʱ��仯ʱ����ϵ�ﵽƽ��
��3����������͵�ˮ�ᷢ�����¶�����Ӧ:
��Ӧ | ��� | |
��һ�� | SO2+I2+2H2O | 9.2kJ��mo1-1 |
�ڶ��� | I2+ I�� | 23.5kJ��mo1-1 |
һ�������£�1mol SO2�ֱ���뵽�����ͬ��Ũ�Ȳ�ͬ�ĵ�ˮ�У���ϵ�ﵽƽ���H+��I3����SO42�������ʵ�����n(I2)/n(SO2)�ı仯������ͼ (���Է�Ӧǰ�������仯)��
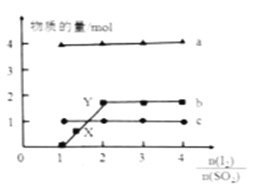
��������ΪX���I��Ũ��С��Y�㣬����Ϊ�ù۵��Ƿ���ȷ________��ԭ����_________________��
�ڵ�n(I2)/n(SO2)=4ʱ,������ͼ������ϵ��n (I��)��Ӧʱ��ı仯���ߡ�________
�ۻ�ѧ��ȤС������������������ⶨI2+I��![]() I3����ƽ�ⳣ�������������½��У�ʵ������Һ����仯���Բ���)��
I3����ƽ�ⳣ�������������½��У�ʵ������Һ����仯���Բ���)��
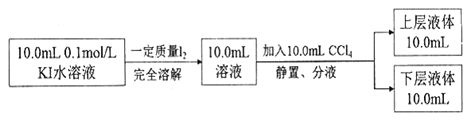
��֪��I����I3 ��������CCl4��:һ���¶��£��ⵥ�������Ȼ�̼��ˮ���Һ���У��ⵥ�ʵ�Ũ�ȱ�ֵ ![]() ����һ������(��Kd��ʾ����Ϊ����ϵ��)������������ Kd=85��ʵ�����ϲ���Һ��c(I3 ��)=0.049mol/L���²�Һ����c(I2)=0.085mol��L-1������������ݣ���������������I2+ I��
����һ������(��Kd��ʾ����Ϊ����ϵ��)������������ Kd=85��ʵ�����ϲ���Һ��c(I3 ��)=0.049mol/L���²�Һ����c(I2)=0.085mol��L-1������������ݣ���������������I2+ I��![]() I3����ƽ�ⳣ��K=_______(������λ��Ч����)��
I3����ƽ�ⳣ��K=_______(������λ��Ч����)��
���𰸡�2NO3����12H����10e��=N2��6H2OBC����ȷͼ��b�߱�ʾI3��������n(I2)/n(SO2)��ֵ����������I2��I��![]() I3��ƽ��������Ӧ�����ƶ���ƽ���I��Ũ�Ƚ���
I3��ƽ��������Ӧ�����ƶ���ƽ���I��Ũ�Ƚ��� 961
961
��������
��1�����ݵ���ԭ���������ϵõ����ӣ�������ԭ��Ӧ������װ��ͼ��NO3���������Ϸŵ磬����ʻ���Ϊ���ԣ����������ӦʽΪ2NO3����12H����10e��=N2��6H2O����2��A��״̬Mʱ���״����������Ϊ10%����������£�����CO�����ʵ���Ϊxmol����ƽ��ʱCO�����ʵ���Ϊ(1��x)mol��������������ʵ���Ϊ(3��2x)mol�������![]() ��100%=10%�����x=7/8����CO��ת����Ϊ7/8����A����B������ͼ�������Ҽ״��������������˵��ƽ��������Ӧ�����ƶ���������������ԭ����ѹǿ��С��ϵ��a<b<c<d����B��ȷ��C������Ϊ���º�ѹ�£��ٳ��������״�����ƽ��Ϊ��Чƽ�⣬�����´ﵽƽ���״�������������䣬��C��ȷ��D����Ϊ�ǰ��ջ�ѧ������Ͷ�룬�ñ�ֵʼ�ձ��ֲ��䣬��D����3�����ݷ�Ӧ���裬�Ƴ�aΪH����BΪI3����CΪSO42����������n(I2)/n(SO2)�����ӣ�˵����ԭ���Ļ���������I2�����ʵ������ڶ�����ƽ��������Ӧ�����ƶ���I����ת���ʽ��ͣ���˸ù۵㲻��ȷ��ԭ����ͼ��b�߱�ʾI3��������n(I2)/n(SO2)��ֵ����������I2��I��
��100%=10%�����x=7/8����CO��ת����Ϊ7/8����A����B������ͼ�������Ҽ״��������������˵��ƽ��������Ӧ�����ƶ���������������ԭ����ѹǿ��С��ϵ��a<b<c<d����B��ȷ��C������Ϊ���º�ѹ�£��ٳ��������״�����ƽ��Ϊ��Чƽ�⣬�����´ﵽƽ���״�������������䣬��C��ȷ��D����Ϊ�ǰ��ջ�ѧ������Ͷ�룬�ñ�ֵʼ�ձ��ֲ��䣬��D����3�����ݷ�Ӧ���裬�Ƴ�aΪH����BΪI3����CΪSO42����������n(I2)/n(SO2)�����ӣ�˵����ԭ���Ļ���������I2�����ʵ������ڶ�����ƽ��������Ӧ�����ƶ���I����ת���ʽ��ͣ���˸ù۵㲻��ȷ��ԭ����ͼ��b�߱�ʾI3��������n(I2)/n(SO2)��ֵ����������I2��I��![]() I3��ƽ��������Ӧ�����ƶ���ƽ���I��Ũ�Ƚ��ͣ�����Ϊ��ʼͨ��SO2Ϊ1mol����n(I2)/n(SO2)=4ʱ��n(I2)=4mol������I�����ʵ������ֵΪ2mol�������ܴﵽ2mol����һ���Ļ��С����Ӧ���ʿ죬�ڶ�����ܴ�Ӧ���ʽ��������ͼ����
I3��ƽ��������Ӧ�����ƶ���ƽ���I��Ũ�Ƚ��ͣ�����Ϊ��ʼͨ��SO2Ϊ1mol����n(I2)/n(SO2)=4ʱ��n(I2)=4mol������I�����ʵ������ֵΪ2mol�������ܴﵽ2mol����һ���Ļ��С����Ӧ���ʿ죬�ڶ�����ܴ�Ӧ���ʽ��������ͼ���� ����ƽ�ⳣ��ֻ���¶ȵ�Ӱ�죬���Ȼ�̼���ܶȴ���ˮ���²�ΪI2(CCl4)���ϲ�ΪI2(H2O)������Kd�Ķ��壬�Ƴ���
����ƽ�ⳣ��ֻ���¶ȵ�Ӱ�죬���Ȼ�̼���ܶȴ���ˮ���²�ΪI2(CCl4)���ϲ�ΪI2(H2O)������Kd�Ķ��壬�Ƴ���![]() =Kd��������ֵ�������c[I2(H2O)]=0.001mol��L��1��10mL��Һ��c(I3��)=0.049mol��L��1�����ʵ���Ϊ0.00049mol����Ӧ��I�����ʵ���Ϊ0.00049mol��ƽ��ʱI�����ʵ���Ϊ(0.1��0.01��0.00049)mol=0.00051mol��L��1����c(I��)=0.051mol��L��1������ƽ�ⳣ���ı���ʽ��K=
=Kd��������ֵ�������c[I2(H2O)]=0.001mol��L��1��10mL��Һ��c(I3��)=0.049mol��L��1�����ʵ���Ϊ0.00049mol����Ӧ��I�����ʵ���Ϊ0.00049mol��ƽ��ʱI�����ʵ���Ϊ(0.1��0.01��0.00049)mol=0.00051mol��L��1����c(I��)=0.051mol��L��1������ƽ�ⳣ���ı���ʽ��K=![]() =961��
=961��