��Ŀ����
ˮ����Ȼ�����ձ���ڵ�һ�����ʣ�����������Ϣ�ش����⣺
��1����֪2H2O=H3O++OH-��H3O+�����幹����______________________________������ԭ�ӵ��ӻ�������__________________��
��2����OH-��H2O��H3O+��H2O2�о����еĻ�ѧ���ǣ��� ��
������ ��A�����Լ��� B���Ǽ��Լ��� C����λ��
��3��s�����s����ص��γɵĹ��ۼ����÷��ű�ʾΪ����s-s��p�����p����ԡ�ͷ��ͷ����ʽ�ص��γɵĹ��ۼ����÷��Ŧ�p-p����H2O���Ӻ��еĹ��ۼ��÷��ű�ʾΪ__________________����
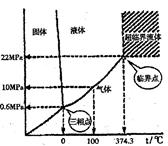
��4����20�棬1.01��105Pa��ˮ���Խ�ɱ�����Ϊ�ȱ������������ۻ�ʱ�����˷�������������롰�ȱ����ۻ�ʱ���˷�������������ȫ��ͬ���ǣ����� ��
�������� A�����ʯ�� B���ɱ��� C��ʳ�Ρ� D����̬��
��1����֪2H2O=H3O++OH-��H3O+�����幹����______________________________������ԭ�ӵ��ӻ�������__________________��
��2����OH-��H2O��H3O+��H2O2�о����еĻ�ѧ���ǣ��� ��
������ ��A�����Լ��� B���Ǽ��Լ��� C����λ��
��3��s�����s����ص��γɵĹ��ۼ����÷��ű�ʾΪ����s-s��p�����p����ԡ�ͷ��ͷ����ʽ�ص��γɵĹ��ۼ����÷��Ŧ�p-p����H2O���Ӻ��еĹ��ۼ��÷��ű�ʾΪ__________________����
��4����20�棬1.01��105Pa��ˮ���Խ�ɱ�����Ϊ�ȱ������������ۻ�ʱ�����˷�������������롰�ȱ����ۻ�ʱ���˷�������������ȫ��ͬ���ǣ����� ��
�������� A�����ʯ�� B���ɱ��� C��ʳ�Ρ� D����̬��
��1��������sp3�� ��2��A�� ��3����s-p�� ��4��D�� ע�����»���+���?
H3O+������ԭ��O����3��ԭ�ӣ���������1�Թ¶Ե��Ӷԣ������γ�4���ӻ�������ӻ�������sp3��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ