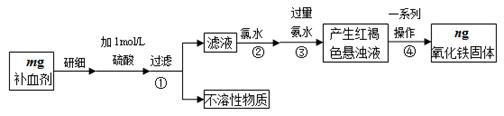
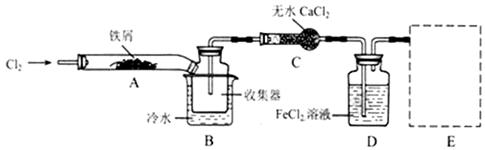
��Ŀ����
ijͬѧ��FeCl3���������Լ�FeSO4�����ȶ��Խ�������̽����
��1����������FeSO4���·ֽ⣬��Ӧ����ʽΪ�� 2FeSO4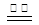 Fe2O3+SO2��+SO3����
Fe2O3+SO2��+SO3����
����˫���ű�����·ֽ�FeSO4�Ʊ�Fe2O3��Ӧ�е���ת�Ƶķ������Ŀ ��
��Ϊ�ռ�SO3����֤SO2��Ӧ����������ͨ����û�� �е�U�ܡ�ϴ��ƿ�е� ��NaOH��Һ��
��2��ʵ��̽��Fe3+�������ԣ���FeCl3��Һ��ͨ��һ������SO2���壬��Һ�ɻ�ɫ��Ϊdz��ɫ��
��dz��ɫ��Һ��һ�����ڵ�������H+��Cl-�� �����ܴ��ڵ����� (��д���)��
A��Fe3+ B��Fe2+ C��SO42- D��H2SO3
��Ϊȷ�Ͽ��ܴ��ڵ�����Ӧѡ����Լ��� (��д���)��
A��ϡ���� B��NaOH��Һ C��KSCN��Һ D��Ʒ����Һ
��1����������FeSO4���·ֽ⣬��Ӧ����ʽΪ�� 2FeSO4
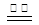 Fe2O3+SO2��+SO3����
Fe2O3+SO2��+SO3��������˫���ű�����·ֽ�FeSO4�Ʊ�Fe2O3��Ӧ�е���ת�Ƶķ������Ŀ ��
��Ϊ�ռ�SO3����֤SO2��Ӧ����������ͨ����û�� �е�U�ܡ�ϴ��ƿ�е� ��NaOH��Һ��
��2��ʵ��̽��Fe3+�������ԣ���FeCl3��Һ��ͨ��һ������SO2���壬��Һ�ɻ�ɫ��Ϊdz��ɫ��
��dz��ɫ��Һ��һ�����ڵ�������H+��Cl-�� �����ܴ��ڵ����� (��д���)��
A��Fe3+ B��Fe2+ C��SO42- D��H2SO3
��Ϊȷ�Ͽ��ܴ��ڵ�����Ӧѡ����Լ��� (��д���)��
A��ϡ���� B��NaOH��Һ C��KSCN��Һ D��Ʒ����Һ
��
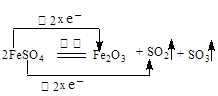 ��2�֣�
��2�֣��� ��ˮ����Ʒ����Һ��������ȷ�𰸣���2�֣���
��2����BC��2�֣��� AD��2�֣���ѡ��һ����1�֣���ѡ0�֣�
��CD��2�֣���ѡ��һ����1�֣���ѡ0�֣�
�����������1��FeSO4���·ֽ�����ΪSO2��SO3���ռ�SO2���ȷ����SO3������SO3�ܷе�߳�����ΪҺ�壬���ñ�ˮ��ȴ���ɵõ�SO3����ʹ�����SO2���壻����SO2һ����Ʒ���Լ�����2����Һ�ɻ�ɫ��Ϊdz��ɫ��˵��Fe3+��SO2���巴Ӧ����Fe2+��SO4����������δ��Fe3+��Ӧ��SO2��ˮ��Ӧ����H2SO3��������������Ϊδ��Ӧ���Fe3+���������������ֱ���Ʒ����Һ��KSCN��Һ��

��ϰ��ϵ�д�
�����Ŀ