��Ŀ����
����Ŀ��A��B��C��D��E��Ϊ�л����������֮��Ĺ�ϵ��ͼ��ʾ
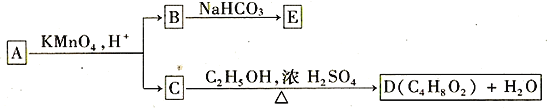
��ʾ����RCH=CHR'�����Ը��������Һ�з�Ӧ����RCOOH��R'COOH������R��R'Ϊ�����
��RCH2OH�����Ը��������Һ�з�Ӧ����RCOOH
�����ԣ�RCOOH > H2CO3
�ش��������⣺
(1)ֱ��������A����Է�������С��90��A������̼����Ԫ�ص�����������Ϊ0.814������Ϊ��Ԫ�أ���A�ķ���ʽΪ__________________��
(2)��֪B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1�U2������Ũ����Ĵ��£�B��������C2H5OH������Ӧ�Ļ�ѧ����ʽ��_________________________________����Ӧ����Ϊ________________��
(3)A��������������÷ų���������ʹ������Ȼ�̼��Һ��ɫ����A�Ľṹ��ʽ��______________��
(4)A���ܷ������ֻ�ѧ��Ӧ��д��A�������з�Ӧ�Ļ�ѧ����ʽ��ע����Ӧ������
�Ӿ۷�Ӧ��_________________________________________
��ȥ��Ӧ��________________________________________________________________
���𰸡�C5H10O HOOC��CH2��COOH + 2C2H5OH![]() C2H5OOC��CH2��COOC2H5 +2H2O ������Ӧ(��ȡ����Ӧ) HO��CH2��CH2��CH=CH��CH3 n HO��CH2��CH2��CH=CH��CH3
C2H5OOC��CH2��COOC2H5 +2H2O ������Ӧ(��ȡ����Ӧ) HO��CH2��CH2��CH=CH��CH3 n HO��CH2��CH2��CH=CH��CH3 ![]()
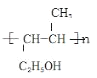 HO��CH2��CH2��CH=CH��CH3
HO��CH2��CH2��CH=CH��CH3 ![]() CH2=CH��CH=CH��CH3 + H2O
CH2=CH��CH=CH��CH3 + H2O
��������
���ݹ�ϵ��C��D����������Ӧ���Ƴ�C�ṹ��ʽΪCH3COOH����������(1)����Ԫ����������Ϊ(1��0.814)=0.186����A�����к����ĸ���Ϊx������x��16/0.186<90���Ƴ�x<1.05����A�����к���1����ԭ�ӣ�A��Ħ������Ϊ1��16/0.186g��mol��1=86g��mol��1��A��������Ħ������Ϊ(86��16)g��mol��1=70g��mol��1��70��12=5��10����A�ķ���ʽΪC5H10O��Ȼ�����ÿ�ʽ��з����ó��ṹ��
��1��Ԫ����������Ϊ(1��0.814)=0.186����A�����к����ĸ���Ϊx������x��16/0.186<90���Ƴ�x<1.05����A�����к���1����ԭ�ӣ�A��Ħ������Ϊ1��16/0.186g��mol��1=86g��mol��1��A��������Ħ������Ϊ(86��16)g��mol��1=70g��mol��1��70��12=5��10����A�ķ���ʽΪC5H10O��
��2��B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2���Ƴ�1��B�����к���2���Ȼ������ݹ�ϵ��C��D����������Ӧ���Ƴ�C�ṹ��ʽΪCH3COOH�� A��Ӧ���С�CH3CH=��������A�ķ���ʽ����1��B��Ӧ����3��C����B�Ľṹ��ʽΪHOOCCH2COOH��B��C2H5OH��Ũ���������·���������Ӧ��ȡ����Ӧ���䷴Ӧ����ʽΪHOOC��CH2��COOH + 2C2H5OH![]() C2H5OOC��CH2��COOC2H5 +2H2O��
C2H5OOC��CH2��COOC2H5 +2H2O��
��3��A��������Ʒ�Ӧ�ų�������A�к����ǻ�����ʹ������Ȼ�̼��ɫ��A�к���̼̼˫�������ݣ�2�����Ƴ�A�Ľṹ��ʽΪCH3CH=CH2CH2OH��
��4��A�к���̼̼˫�����ܷ����Ӿ۷�Ӧ���䷴Ӧ����ʽΪn HO��CH2��CH2��CH=CH��CH3 ![]()
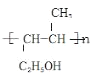 ���ǻ�����̼ԭ�ӵ����ڵ�̼�����⣬�ܷ�����ȥ��Ӧ���䷴Ӧ����ʽΪ��HO��CH2��CH2��CH=CH��CH3
���ǻ�����̼ԭ�ӵ����ڵ�̼�����⣬�ܷ�����ȥ��Ӧ���䷴Ӧ����ʽΪ��HO��CH2��CH2��CH=CH��CH3 ![]() CH2=CH��CH=CH��CH3 + H2O��
CH2=CH��CH=CH��CH3 + H2O��

����Ŀ��ij����С�����ʵ������ȡ���ᴿ���������ķ������£�
��֪�����Ȼ��ƿ����Ҵ��γ�CaCl2��6C2H5OH
���й��л���ķе㣺
�Լ� | ���� | �Ҵ� | ���� | �������� |
�е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��2CH3CH2OH![]() CH3CH2OCH2CH3��H2O
CH3CH2OCH2CH3��H2O
I���Ʊ�����: װ����ͼ��ʾ��A�з���Ũ���ᣬB�з���9.5mL��ˮ�Ҵ���6mL�����ᣬD�з��б���̼������Һ��
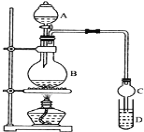
(1)ʵ������еμӴ�Լ3mLŨ���ᣬB���ݻ�����ʵ���________(������ȷѡ��ǰ����ĸ)
A��25mL B��50mL C��250mL D��500mL
(2)���θ���ܵ���Ҫ������_________________________��
(3)����Na2CO3��Һ��������_______________________________________________________
II���ᴿ�������ٽ�D�л��Һ���з��롣
���л�����5mL����ʳ��ˮϴ�ӣ�����5mL�����Ȼ�����Һϴ�ӣ������ˮϴ�ӡ��л��㵹��һ�������ƿ�У�����ˮ����þ����ôֲ��
�۽��ֲ��������ռ�77.1�����֣��õ��������������������
(4)�ڢٲ�������Һʱ��ѡ�õ���Ҫ����������������_________________��
(5)�ڢڲ����ñ���ʳ��ˮϴȥ̼���ƺ����ñ����Ȼ�����Һ�������ˮϴ�ӣ��ֱ���Ҫϴȥ�ֲ�Ʒ�е�__________________��______________��