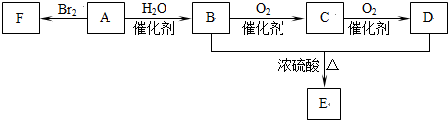
��Ŀ����
11����ͭ����������Cu��Zn��Ni��CuO��ZnO��������FeO��Fe2O3�ȣ���������ȡ����ͭ��п�κ�������泥��������������£�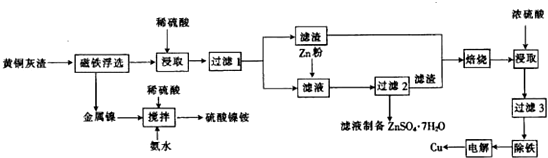
�ش��������⣺
��1����ȡʱ��Fe2O3��ϡ���ᷴӦ�����ӷ���ʽΪFe2O3+6H+=2Fe3++3H2O������Zn�ۣ���Ҫ����ԭ����������H+��Fe3+��Cu2+
��2������Ũ������ν�ȡ���ܲ������ж�������SO2�����ķ������ð�ˮ����
��3������ʱ����Ҫ����˫��ˮ��������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O���ٵ�����Һʱ��pHʹ����Fe��OH��3���ѧʽ������ʽ����ȥ
��4�����ʱ�������ĵ缫��ӦʽΪCu2++2e-=Cu�������õ�����Һ������ķ����Ǽ���ZnO��Ӧ����������2����Һ�ϲ��Ʊ�ZnSO4•7H2O
��5���������[��NH4��xNiy��SO4��m•nH2O]�dz��õĻ�ѧ�����Լ���ijͬѧΪ�ⶨ������淋���ɣ���������ʵ���ȷ��ȡ1.9750g����Ʒ�����Ƴ�100.00mL����ҺA����ȷ��ȡ25.00mL����ҺA����0.04000mol•L-1��EDTA��Na2H2Y������Һ�ζ����е�Ni2+�����ӷ���ʽΪNi2++H2Y2-=NiY2-+2H+��������EDTA������Һ31.25mL������ȡ25.00mL��ҺA����������NaOH����Һ����ּ��ȣ�����NH3��56.00mL����״������
��������淋Ļ�ѧʽΪ��NH4��2Ni��SO4��2•6H2O
�����ⶨ����ʹ��ǰδ��EDTA����Һˢϴ�����õ�N2+������ƫ�ߣ��ƫ�ߡ���ƫ�͡����䡱��
���� ��ͭ���м���ϡ���ᣬϡ�����Zn��CuO��ZnO��FeO��Fe2O3��Ӧ������1Ϊͭ��������SiO2����Һ1��Ҫ����Zn2+��Cu2+��Fe2+��H+������п�ۣ�п�ۿ���Cu2+��Fe2+��H+��Ӧ����ͭ�������ʣ�����2��ҪΪͭ�������ʺ�����п�����ձ��ڳ�ȥͭ���ڲ�������п�����ʣ�Ũ������ͭ��Ӧ��������ͭ�Ͷ���������������ð�ˮ���գ��������ͭ��Һ�ɵõ�ͭ���ʣ�п��������Һ���ŵ磬����ZnO�кͺ�����Һ2�ϲ��Ʊ�ZnSO4•7H2O��
��1����ȡʱ��Fe2O3��ϡ���ᷴӦ������������ˮ������п�ۻ�ԭ�����Ӻ�ͭ���ӣ�
��2��Ũ���ᱻ��ԭ�����ж���������������ö�����������������������ð�ˮ���գ�
��3����������������Һ��������������Ϊ�����ӣ�������ҺPHʹ������ȫ��������ȥ��
��4�����ʱ��Һ��ͭ�����������õ���������ͭ���������õ�����Һ������ķ�������ZnO��Ӧ����������2����Һ�ϲ��Ʊ�����п���壬�������ԭ�ϣ�
��5���ٸ��ݷ�Ӧ�͵ζ����ݼ���������ӵ����ʵ��������ݰ����������������������ʵ����������������������������������ʵ��������������������ˮ�����ʵ�����������������淋Ļ�ѧʽ��
�ڵζ���û����ϴ�����±�ҺŨ�ȼ�С���ⶨ�����ƫ�ߣ�
��� �⣺��ͭ���м���ϡ���ᣬϡ�����Zn��CuO��ZnO��FeO��Fe2O3��Ӧ������1Ϊͭ��������SiO2����Һ1��Ҫ����Zn2+��Cu2+��Fe2+��H+������п�ۣ�п�ۿ���Cu2+��Fe2+��H+��Ӧ����ͭ�������ʣ�����2��ҪΪͭ�������ʺ�����п�����ձ��ڳ�ȥͭ���ڲ�������п�����ʣ�Ũ������ͭ��Ӧ��������ͭ�Ͷ���������������ð�ˮ���գ��������ͭ��Һ�ɵõ�ͭ���ʣ�п��������Һ���ŵ磬����ZnO�кͺ�����Һ2�ϲ��Ʊ�ZnSO4•7H2O��
��1����ȡʱ��Fe2O3��ϡ���ᷴӦ������������ˮ����Ӧ�����ӷ���ʽΪ��Fe2O3+6H+=2Fe3++3H2O������п�ۻ�ԭ��Һ�������Ӻ�ͭ���ӣ�
�ʴ�Ϊ��Fe2O3+6H+=2Fe3++3H2O��Fe3+��Cu2+��
��2��Ũ���ᱻ��ԭ�����ж�����������������������ö�����������������������ð�ˮ���գ�
�ʴ�Ϊ��SO2���ð�ˮ���գ�
��3����������������Һ��������������Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��������ҺPHʹ�������γ���������ȫ��������ȥ��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��Fe��OH��3��
��4�����ʱ��Һ��ͭ�����������õ���������ͭ���缫��ӦΪ��Cu2++2e-=Cu���������õ�����Һ������ķ�������ZnO��Ӧ����������2����Һ�ϲ��Ʊ�����п���壬�������ԭ�ϣ�
�ʴ�Ϊ��Cu2++2e-=Cu������ZnO��Ӧ����������2����Һ�ϲ��Ʊ�ZnSO4•7H2O��
��5����25mL��Һ�������ӵ����ʵ����ǣ�n��Ni2+��=n��H2Y2-��=0.04000 mol•L-1��0.03125L=1.250��10-3 mol��
���������ʵ�����������ӵ����ʵ�����n��NH4+��=$\frac{0.056L}{22.4L/mol}$=2.500��10-3 mol
���ݵ���غ㣬����������ʵ����ǣ�n��SO42-��=$\frac{1}{2}$��[2n��Ni2+��+n��NH4+��]=2.500��10-3 mol��
���ԣ�m��Ni2+��=59 g•mol-1��1.250��10-3 mol=0.07375 g
m��NH4+��=18 g•mol-1��2.500��10-3 mol=0.04500 g
m��SO42-��=96 g•mol-1��2.500��10-3 mol=0.2400 g
n��H2O��=$\frac{1.9750g��\frac{25}{100}-��0.07375g+0.04500g+0.2400g��}{18g/mol}$=7.250��10-3 mol
x��y��m��n=n��NH4+����n��Ni2+����n��SO42-����n��H2O��=2��1��2��6��
������淋Ļ�ѧʽΪ��NH4��2Ni��SO4��2•6H2O��
��������淋Ļ�ѧʽ�ǣ�NH4��2Ni��SO4��2•6H2O��
������û���ñ�Һ��ϴ�ζ��ܣ�ʹ�õζ����еı�ҺŨ�ȼ�С���ζ������ĵı�Һ������ⶨ���ƫ�ߣ�
�ʴ��ǣ�ƫ�ߣ�
���� ���⿼���Ʊ�ʵ�鷽������ƣ�Ϊ��Ƶ���㣬�����Ʊ�ԭ����ʵ�������еķ�Ӧ�����ʵ����ʵ�Ϊ���Ĺؼ������ط�����ʵ�顢�����������ۺϿ��飬��Ŀ�Ѷ��еȣ�

| A�� | �ۢܢ� | B�� | �ڢۢ� | C�� | �٢ڢ� | D�� | �٢ڢۢܢ� |
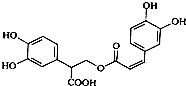
| A�� | �Ե��������ڷ����� | |
| B�� | 1mol�Ե���������ܺͺ�6mol H2�����ӳɷ�Ӧ | |
| C�� | �Ե�������Է���ˮ�ⷴӦ����ȥ��Ӧ��������Ӧ | |
| D�� | 1mol�Ե���������ܺͺ�6mol NaOH��ˮ��Һ��ȫ��Ӧ |
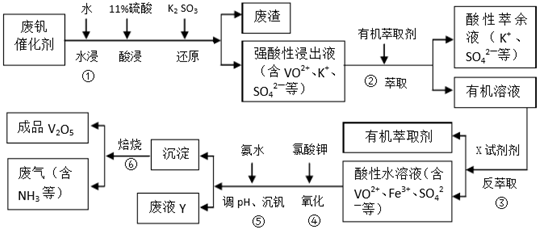
�ش��������⣺
��1�����з�������Ҫ�ɷ���SiO2��
��2���ڣ����еı仯���̿ɼ�Ϊ����ʽ�е�R��ʾVO2+��Fe3+��HA��ʾ�л���ȡ������Ҫ�ɷ֣���R2��SO4��n��ˮ�㣩+2nHA���л��㣩?2RA���л��㣩+nH2SO4��ˮ�㣩��������ȡʱ��������������ԭ���Ǽ�����к�����ʹƽ�����ơ����������ȡ����߷�����ȡ�ʣ�ʵ���ҽ�����ȡ������Ҫʹ�õIJ�������Ϊ��Һ©�����ձ���
��3����ɢ��з�Ӧ�����ӷ���ʽ��1ClO3-6VO2++6H+=6VO3+1Cl-+3H2O
��4��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮��Ĺ�ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������/% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.6 | 96.4 | 93.1 | 89.3 |
��һ���Ʊ�̼��������
��һ�������Ʊ�������������Һ������̼�������Һ��ϲ����������������壮
��1��д�����ӷ���ʽ��Fe2++2HCO3-=FeCO3��+CO2��+H2O��
������̽��̼�����������ȶ��ԣ���������ʡ�ԣ�
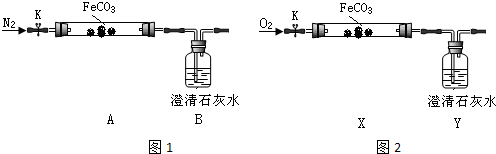
����������װҩƷ����K����ͨ��һ��ʱ�䵪����Ȼ���þƾ������A�����Ȳ����ܣ��۲�Bƿ��Һ����ǣ�������ֽ������ͨ�뵪������������ȴ��
��2����ͨ�뵪����Ŀ�����ž�װ���ڿ����������������ţ�Bƿ������˵���ֽ�����ж�����̼��
��3��ֹͣ����֮ǰ���Ƿ��A��B֮���ܣ��𣺷�������ͨ�뵪��������������ѹ�����С��
������̽��̼��������ԭ��
���������ϡ�
������������һ�ֺ�ɫ��ĩ�������ȶ����ڿ����м��ȣ���Ѹ�ٱ�������������������
��̼�������ڿ���������������������
��4��̽��̼��������������Ӧ�Ĺ���ɷ֣�
�١�������롿
����1 ����ɷ�����������
����2 ����ɷ���������������
����3��������������������
�ڡ�ʵ����֤������������װҩƷ����ֹˮ��K��ͨ�����������Ȳ����ܣ�Yƿ�г���ʯ��ˮ����������ʱ��ֹͣ���ȣ�����ͨ����������������ȴ��
ȡ������������������Թܣ��μ�ϡ���ᣬ�ȣ�������ȫ�ܽ⣮����Һ�ֳɼס���������Һ�����к���ʵ�飮
| ʵ���� | ʵ�鲽�� | ʵ������ |
| i | �����Һ�μ�KSCN��Һ | ��Һ���ɫ |
| ii | ������Һ�μ����Ը��������Һ | ��Һ����ɫ |
��ʵ����ۡ���������ʵ��������С��õ�̼�������������и������յõ���������Һ���ɫ��ֻ֤����+3���������������������������������������������������������
��5������̽����ȡ23.2g��������̼���������ڿ����и������������أ��Ƶù�����������7.2g��ͨ������ȷ������ɷ�����������
| A�� | ��ѧ��Ӧ�����ʱ仯��ʵ���Ǿɻ�ѧ���Ķ��Ѻ��»�ѧ�����γ� | |
| B�� | ���ӻ�������һ���н���Ԫ�غͷǽ���Ԫ�� | |
| C�� | ȫ���ɷǽ���Ԫ���γɵĻ�����һ���ǹ��ۻ����� | |
| D�� | ���ۻ������и�ԭ�Ӷ�һ�����������8�����ȶ��ṹ |
| A�� | ��pH=1����ɫ��Һ�У�SO42-��Cu2+��Na+��Cl- | |
| B�� | ʹpH��ֽ�ʺ�ɫ����Һ�У�Fe2+��NO3-��SO42-��Na+ | |
| C�� | �������ۺ����������������Һ�У�NH4+��Na+��NO3-��SO42- | |
| D�� | ��$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1��1013����Һ�У�NH4+��Ca2+��C1-��K+ |
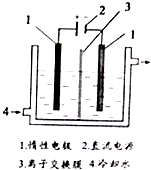 �ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺
�ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺