��Ŀ����
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�EԪ���ǵ�������Ԫ����δ�ɶԵ���������Ԫ�أ�ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1��1mol�������������AgNO3��Һ��Ӧ����������3molAgCl��Fԭ�ӵ�һ�ֺ��ص�������Ϊ65��������Ϊ 36�����������������ش��������⣺������ʱҪ��Ԫ�ط��ű�ʾ��
��1��B�⻯����HCl��Ӧ���ɵĺ���BԪ�����ӵĿռ乹���� .FԪ��ԭ�ӵ�����������Ϊ ����
��2��B3-���ӷֱ���AC2����B��C��ɵ���̬�����ﻥΪ�ȵ����壬��B��C��ɵĻ����ﻯѧʽΪ ��B3-���ӻ����Ժ�һ�������ӻ�Ϊ�ȵ����壬�������ӵ���ʽΪ �����������ӳ����ڼ����ճ������е�һ�ֽ��������ӣ�����������ӷ���Ϊ
��3��A��B��C�ĵ�һ��������С�����˳��Ϊ
��4��E3+�ĺ�������Ų�ʽ�� ��ECl3�γɵ�����λ������ﻯѧʽΪ ��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
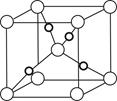
��6����F��+1��������ľ����ṹ��ͼ��FΪ���������ڡ����ס���
��1��B�⻯����HCl��Ӧ���ɵĺ���BԪ�����ӵĿռ乹���� .FԪ��ԭ�ӵ�����������Ϊ ����
��2��B3-���ӷֱ���AC2����B��C��ɵ���̬�����ﻥΪ�ȵ����壬��B��C��ɵĻ����ﻯѧʽΪ ��B3-���ӻ����Ժ�һ�������ӻ�Ϊ�ȵ����壬�������ӵ���ʽΪ �����������ӳ����ڼ����ճ������е�һ�ֽ��������ӣ�����������ӷ���Ϊ
��3��A��B��C�ĵ�һ��������С�����˳��Ϊ
��4��E3+�ĺ�������Ų�ʽ�� ��ECl3�γɵ�����λ������ﻯѧʽΪ ��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
��6����F��+1��������ľ����ṹ��ͼ��FΪ���������ڡ����ס���

(15�֣���1������������(1��) 1 (1��) ��2��N2O(1��) 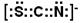 (1��) Fe3+ (1��)
(1��) Fe3+ (1��)
��3��C��O��N (2��) ��4��1s22s22p63s23p63d3 (2��) [Cr(NH3)4(H2O)2]Cl3 (2��)
��5��4Mg��10HNO3��4Mg(NO3)2��NH4NO3��3H2O (2��) ��6���� ( 2��)
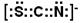 (1��) Fe3+ (1��)
(1��) Fe3+ (1��)��3��C��O��N (2��) ��4��1s22s22p63s23p63d3 (2��) [Cr(NH3)4(H2O)2]Cl3 (2��)
��5��4Mg��10HNO3��4Mg(NO3)2��NH4NO3��3H2O (2��) ��6���� ( 2��)
���������A��B��C��ͬһ���ڵķǽ���Ԫ��, AC2Ϊ�Ǽ��Է��ӣ����A��̼Ԫ�أ�C����Ԫ�ء�B��ԭ����������A��C���ۣ�����B�ǵ�Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ������D��þԪ�ء�EԪ���ǵ�������Ԫ����δ�ɶԵ���������Ԫ�أ���E�Ǹ�Ԫ�ء�Fԭ�ӵ�һ�ֺ��ص�������Ϊ65��������Ϊ 36����F��ԭ��������65��36��29����F��ͭԪ�ء�
��1��B�⻯���ǰ�������HCl��Ӧ���ɵĺ���BԪ��������NH4������ռ乹�������������ͣ����ݹ���ԭ����֪��ͭԭ�ӵ�����������4s1������������������1����
��2��ԭ�����ͼ۵������ֱ���ȵ��ǵȵ����壬B3-���Ӻ���3��5��1��16���۵��ӣ�����B3-���ӷֱ���AC2����B��C��ɵ���̬�����ﻥΪ�ȵ����壬��B��C��ɵĻ����ﻯѧʽΪN2O��B3-���ӻ����Ժ�һ�������ӻ�Ϊ�ȵ����壬����������Ӧ����SCN�����ӣ������ʽΪ
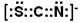 ���������ӳ����ڼ���Fe3����
���������ӳ����ڼ���Fe3������3��ͬ���ڵ�һ������������Ҿ����������ƣ����Ե�һ������O��C�����ڵ�Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܴ�������Ԫ�أ�����A��B��C�ĵ�һ��������С�����˳��ΪC��O��N��
��4�����ݹ���ԭ����֪��E3+�ĺ�������Ų�ʽ��1s22s22p63s23p63d3��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1��1mol�������������AgNO3��Һ��Ӧ����������3molAgCl����˵�������Ӳ�������λ�����γɡ�����CrԪ�صĻ��ϼ��ǣ�3�ۣ��������Ը���λ�������к���6�����壬���а�����4����ˮ��2������˸���λ������Ļ�ѧʽ��[Cr(NH3)4(H2O)2]Cl3��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ����ڵ�Ԫ�ص���ͼ��ǣ�3�ۣ���˵����ԭ����������泥����Ը÷�Ӧ�Ļ�ѧ����ʽ��4Mg��10HNO3��4Mg(NO3)2��NH4NO3��3H2O��
��6�����ݾ����Ľṹ����Ͼ�̯����֪�����������8��
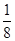 ��1��2�������������4���������ߵĸ���֮����1:2������ΪF��+1����������Cu2O������F�Ǻ���
��1��2�������������4���������ߵĸ���֮����1:2������ΪF��+1����������Cu2O������F�Ǻ���
��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ




 U3++3H2O��ƽ�ⳣ��K= ��
U3++3H2O��ƽ�ⳣ��K= ��