��Ŀ����
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣺
��1����֪BA5Ϊ���ӻ����д�������ʽ__________________________________��
��2��B�Ļ�̬ԭ����������ߵĵ��ӣ���������ڿռ��Т�_________����չ����ԭ�ӹ���ʢ�__________________�Ρ�
��3��ijͬѧ����������Ϣ���ƶ�C�Ļ�̬ԭ�ӵĺ�������Ų�ͼΪ��
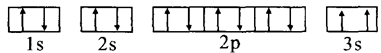
��ͬѧ�����ĵ����Ų�ͼΥ����_________________________��
��4��Gλ�ڢ�_________���________�����۵����Ų�ʽΪ��______________________��
��5��DE3�����У�����ԭ�ӵ��ӻ���ʽΪ��_____________������ӵ����幹��Ϊ��_____________��
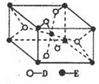
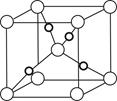
��6��F����ľ�������ͼ��ʾ������þ������ܶ�Ϊag��cm-3�������ӵ�����ΪNA��Fԭ�ӵ�Ħ������ΪM����Fԭ�ӵİ뾶Ϊ_____________cm��
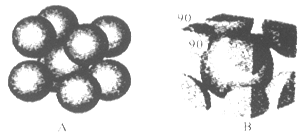
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�ء� |
| BԪ��ԭ�ӵĺ���p��������s��������1�� |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ�I1=738kJ/mol I2=1451J/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ��������� |
| EԪ�ص������������������IJ�Ϊ4�� |
| F��ǰ�������е縺����С��Ԫ�ء� |
| G�����ڱ��ĵ����С� |
��1����֪BA5Ϊ���ӻ����д�������ʽ__________________________________��
��2��B�Ļ�̬ԭ����������ߵĵ��ӣ���������ڿռ��Т�_________����չ����ԭ�ӹ���ʢ�__________________�Ρ�
��3��ijͬѧ����������Ϣ���ƶ�C�Ļ�̬ԭ�ӵĺ�������Ų�ͼΪ��

��ͬѧ�����ĵ����Ų�ͼΥ����_________________________��
��4��Gλ�ڢ�_________���________�����۵����Ų�ʽΪ��______________________��
��5��DE3�����У�����ԭ�ӵ��ӻ���ʽΪ��_____________������ӵ����幹��Ϊ��_____________��
��6��F����ľ�������ͼ��ʾ������þ������ܶ�Ϊag��cm-3�������ӵ�����ΪNA��Fԭ�ӵ�Ħ������ΪM����Fԭ�ӵİ뾶Ϊ_____________cm��
(1)
 (2��) ��2��3(2��) ��������(2��) ��3������ԭ��(1��) ��4����VIIB(1��)
(2��) ��2��3(2��) ��������(2��) ��3������ԭ��(1��) ��4����VIIB(1��) d(1��) ��3d54s2(1��) (5)sp3(1��) ������(1��) ��6��
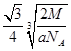 (3��)
(3��)���������������Ŀ������Ϣ��֪��AΪHԪ�ء�BԪ�صĺ�������Ų�Ϊ1s22s22p3��������Ϊ1s22s22p53s2��
����BΪNԪ�ء�����Cԭ�ӵĵ�һ�����ĵ����ܵ����ݣ���֪Cԭ�������2��������ʧȥ��������
��Ϣ������Ԫ�أ�ԭ����������������֪CΪMgԪ�ء�Dԭ�Ӻ�������p���ȫ�������������֪D��
��������Ų�Ϊ1s22s22p63s23p3����Ϊ1s22s22p63s23p6��ǰ���Ų���PԪ�ء�������ArԪ�أ�������Ԫ�أ���EԪ�ص������������������IJ�Ϊ4��D��PԪ�أ�����Eֻ���ڵ������ڣ�����Ϊ��VIIA�塣EΪClԪ�ء�F��ǰ�������е縺����С��Ԫ�أ��縺��ͬ���ڴ�����������ͬ������ϵ�����С�����Կ���֪F��KԪ�ء�G�����ڱ��ĵ����С�����Ϊ��VIIB�塣ΪMnԪ�ء�������������֪��Ԫ�طֱ�Ϊ��A��H��B��N��C��Mg��D��P��E��Cl��F��K��G��Mn��
��1����֪NH5Ϊ���ӻ�����ǽ������ӻ������ȻӦ����Σ�����笠����ӣ����������ʽΪ
 ��
����2��N�ĺ�������Ų�Ϊ1s22s22p3�����̬ԭ����������ߵĵ���Ӧ��p���ӣ���������ڿռ���3����չ����x��y��z����ԭ�ӹ���������Ρ�
��3��ijͬѧ����������Ϣ���ƶ�C�Ļ�̬ԭ�ӵĺ�������Ų�ͼΪ��

�����Ų�ͼ��3sΥ��������ԭ����ͬһ����ϲ����ܴ�������״̬��ȫһ���ĵ��ӡ�
��4��Mn�۵����Ų�ʽΪ3d54s2��λ�ڵ�VIIB��d����
��5��PCl3�����У�����VSEPR���ۣ��۲���Ӷ���ĿΪ4�ԣ��µ��Ӷ�����1����������ԭ�ӵ��ӻ���ʽΪsp3������ӵ����幹��Ϊ������
��6��F����ľ�������ͼ��ʾ������þ������ܶ�Ϊag��cm-3�������ӵ�����ΪNA��Fԭ�ӵ�Ħ������ΪM�����ݾ����ṹ���ƶϳ���һ��������Ԫ�к���2������ԭ�ӡ��辧���߳�Ϊbcm������
 b=4r,���ݦ�=
b=4r,���ݦ�=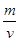 �����У�
������ ��3=
��3= ���Ƴ�r=
���Ƴ�r=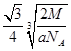 ��
��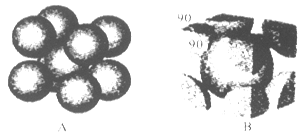

��ϰ��ϵ�д�
�����Ŀ