��Ŀ����
ȫ����ÿ����������ʧ�ĸ���Լռ��������������1/4��ijѧ����̽��������������������ɾ��������ֱ�ͬʱ����A��B��C��֧�Թ��н����о���
��1�����������ѧ���������ʵ����Ƶ����ݣ�
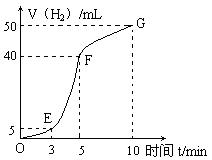
��2��һ�ܺ��Ϊ___________���Թ��е������������⡣
��3������ͬ����������������ͬʱ�������������ˣ���װ���Ϸ��Ȱ�װ�ڱ����������⣬��ԭ����_____________________________________________��
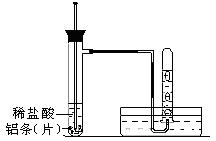
��4���ں�������ʱ��Ϊ��ʹ�ӿڸ����ι̣�����ϡ������ϴ�ӿڴ������⣬�÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
��1�����������ѧ���������ʵ����Ƶ����ݣ�
| ��� | �������� | ʵ��Ŀ�� |
| A | | ̽�����ڸ�������е�������� |
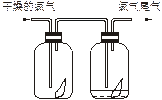
| B | ����������ע������ˮ��û����������ֲ����Һ�� | |
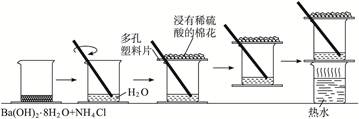
| C | | ̽�������п�����ˮ��ʱ��������� |
��3������ͬ����������������ͬʱ�������������ˣ���װ���Ϸ��Ȱ�װ�ڱ����������⣬��ԭ����_____________________________________________��
��4���ں�������ʱ��Ϊ��ʹ�ӿڸ����ι̣�����ϡ������ϴ�ӿڴ������⣬�÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
��1�����Ⱥ���Թܣ�С�ķ����������������� ̽��������ˮ��������������ʱ��������� С�ķ���������ע������ˮ��ʹ�������ֽ���ˮ��
��2��C
��3���Ϸ��ȱ���������ʪ������������
��4��Fe2O3+6HCl====2FeCl3+3H2O
��2��C
��3���Ϸ��ȱ���������ʪ������������
��4��Fe2O3+6HCl====2FeCl3+3H2O
���ⲻ���������������������Ҳ�������о�������Ĺ��̡���������������ˮ�йأ����Կ���������������������Ա��������֤������1������ֻ���������Ӵ�����2������ֻ��ˮ�Ӵ�����3����������ˮ��������Ӵ��������ϵ���֪ʶ�����˲�ͬ����λ�á������������������Ӱ�죬�������о�����ʴ������ʵ�ʼ�ֵ��

��ϰ��ϵ�д�
�����Ŀ