��Ŀ����
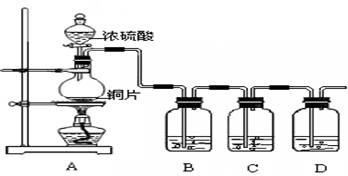
ijУѧ��������ͼ��ʾװ�ý���ʵ�飬��̽�������巢����Ӧ��ԭ���������ᴿ��Ӧ�IJ��

��ش��������⣺
��1��д��II�з�Ӧ�Ļ�ѧ����ʽ ��
��2���۲쵽II�е������� ��
��3��ʵ�鿪ʼʱ���ر�K2������K1�ͷ�Һ©���������μӱ���Һ��Ļ��Һ����Ӧ��ʼ��III��С�Թ��ڱ��������� ��
��4����˵������Һ�巢����ȡ����Ӧ�������� ��
��5����������ƿ�ڷ�Ӧ���Һ�����ν�������ʵ������Ϳɵõ��ϴ������屽��
��������ˮϴ�ӣ�����Һ������5%��NaOH��Һϴ�ӣ�����Һ��
��������ˮϴ�ӣ�����Һ���ܼ�����ˮCaCl2��ĩ���
�� ����������ƣ���

��ش��������⣺
��1��д��II�з�Ӧ�Ļ�ѧ����ʽ ��
��2���۲쵽II�е������� ��
��3��ʵ�鿪ʼʱ���ر�K2������K1�ͷ�Һ©���������μӱ���Һ��Ļ��Һ����Ӧ��ʼ��III��С�Թ��ڱ��������� ��
��4����˵������Һ�巢����ȡ����Ӧ�������� ��
��5����������ƿ�ڷ�Ӧ���Һ�����ν�������ʵ������Ϳɵõ��ϴ������屽��
��������ˮϴ�ӣ�����Һ������5%��NaOH��Һϴ�ӣ�����Һ��
��������ˮϴ�ӣ�����Һ���ܼ�����ˮCaCl2��ĩ���
�� ����������ƣ���
��

��ϰ��ϵ�д�
�����Ŀ
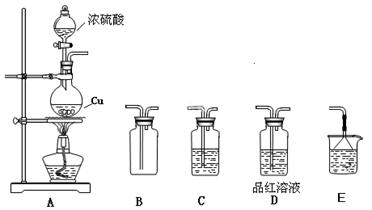
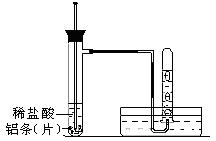
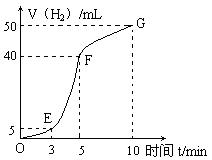
 ����������ʵ��̽����
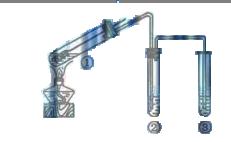
����������ʵ��̽����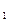 g�û�������Ʒ�������в������������������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ�˿�������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��
g�û�������Ʒ�������в������������������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ�˿�������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��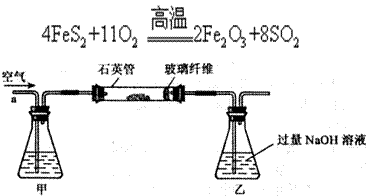
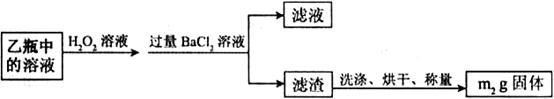
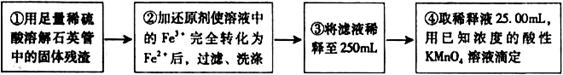
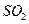 ����
���� ���ɣ�����м�ˮ���۲���ɫ
���ɣ�����м�ˮ���۲���ɫ