��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��е�![]() Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش��������⣺
Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش��������⣺
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 | |
�� |
|
| ||||||
�� |
|
|
|
|
|
| ||
�� |
|
|
(1)��ѧ��������õ�Ԫ��ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ________��
(2)Ԫ��![]() ��
��![]() �ļ��⻯���е��ȶ�����ǿ����______________
�ļ��⻯���е��ȶ�����ǿ����______________![]() �û�ѧʽ��ʾ
�û�ѧʽ��ʾ![]() ��
��
(3)Ԫ�ص�����������Ӧ��ˮ������������ǿ����________��������ǿ����_______�������Ե�����������_______________��Ԫ��![]() ������������Ӧˮ�����к��еĻ�ѧ������Ϊ________________��
������������Ӧˮ�����к��еĻ�ѧ������Ϊ________________��
(4)��![]() ����Ԫ���У������Ӱ뾶��С����_________��
����Ԫ���У������Ӱ뾶��С����_________��
(5)![]() �ĵ���ʽΪ___________��
�ĵ���ʽΪ___________��![]() �ĵ���ʽΪ_________��
�ĵ���ʽΪ_________��
(6)��![]() ��
��![]() �ĵ����У������Խ�ǿ����__________���û�ѧ��Ӧ����ʽ֤����___________________________��
�ĵ����У������Խ�ǿ����__________���û�ѧ��Ӧ����ʽ֤����___________________________��
���𰸡�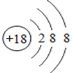 HF HClO4 KOH Al(OH)3 ���Ӽ����ۼ� Al3+
HF HClO4 KOH Al(OH)3 ���Ӽ����ۼ� Al3+ ![]()
![]() Cl2 Cl2+2Br-=2Cl-+Br2
Cl2 Cl2+2Br-=2Cl-+Br2
��������
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK����ΪBr����϶�Ӧ���ʡ�������������Լ�Ԫ��������֪ʶ�����⣮
��1��ԭ�ӽṹ�����������8�����ȶ��ṹ�Ļ�ѧ��������ã���ֻ��Ar������������Ϊ8����������ã���Ar�ĺ˵����Ϊ18��������Ϊ18�����������Ϊ18�������Ӳ��ϵ������ֱ�Ϊ2��8��8����ԭ�ӽṹʾ��ͼΪ ����Ϊ
����Ϊ ��
��
��2����ΪNԪ�أ���ΪFԪ�أ���������ͬ����Ԫ�أ������ң��ǽ�������ǿ���ǽ�����F>N���ǽ�����Խǿ����̬�⻯��Խ�ȶ������ȶ���HF>NH3����ΪHF��
��3��������Խǿ������������Ӧ��ˮ����ļ���Խǿ���ǽ�����Խǿ������������Ӧ��ˮ����������Խǿ������FԪ�������ۣ�����Ԫ�������ɣ�������Ԫ��������������Ӧ��ˮ����������ǿ��ΪHClO4��K�Ľ�������ǿ������������Ӧ��ˮ����ļ�����ǿ��ΪKOH�������Ե�����������Al(OH)3����ΪNaԪ�أ�������������Ӧˮ����ΪNaOH������ʽΪ![]() ���ṹ�к������Ӽ����ۼ�����ΪHClO4��KOH��Al(OH)3�����Ӽ����ۼ���
���ṹ�к������Ӽ����ۼ�����ΪHClO4��KOH��Al(OH)3�����Ӽ����ۼ���
��4����ΪNaԪ�أ���ΪMgԪ�أ���ΪAlԪ�أ���ΪClԪ�أ��γɼ����ӣ�Na+��Mg2+��Al3+���Ӳ�ֻ�ж��㣬Cl-�����㣬��Na+��Mg2+��Al3+���Ǿ�����ͬ�ĵ��Ӳ�ṹ�����ݵ��Ӳ���Խ�࣬�뾶Խ������ͬ���Ӳ�ṹ�����ӣ�ԭ������Խ�뾶ԽС��������������Ӱ뾶��С��ΪAl3+����ΪAl3+��
��5��Na2O2����������������γ����Ӽ�����ԭ������ԭ���γɹ��ۼ��������ʽΪ![]() ��CO2��Cԭ����ÿ��Oԭ���γ����Թ��õ��Ӷԣ������ʽΪ
��CO2��Cԭ����ÿ��Oԭ���γ����Թ��õ��Ӷԣ������ʽΪ![]() ����Ϊ
����Ϊ![]() ��
��![]() ��
��
��6����ΪClԪ�أ���ΪBr����������ͬ����Ԫ�أ��������£��ǽ��������μ�������ǽ�����Cl>Br���ǽ�����Խǿ���䵥�ʵ�������Խǿ��������Cl2>Br2����Cl2�û���Br2�Ϳ�֤������ѧ��Ӧ����ʽΪCl2+2Br-=2Cl-+Br2����ΪCl2��Cl2+2Br-=2Cl-+Br2��

 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�