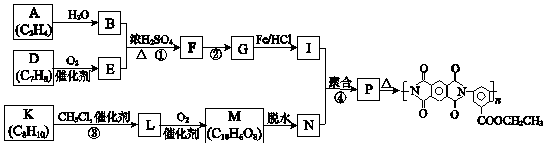
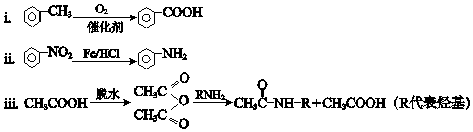��Ŀ����
����Ŀ����ʽ̼��þ���������ࡢҽҩ�ͻ�ױƷ�ȹ�ҵ����ѧʽΪ4MgCO3Mg ��OH��25H2O��ij��ʽ̼��þ�к���SiO2���ʣ�Ϊ�ⶨ�䴿�ȣ�ij��ȤС����������·����� 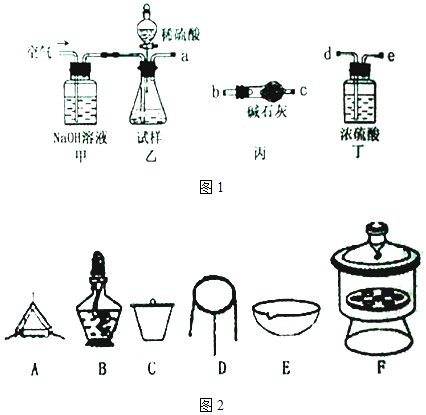
����I ȡһ����������Ʒ���������ַ�Ӧ��ͨ���ⶨ����CO2���������㴿��
��1�����з�����Ӧ�ķ���ʽΪ ��
��2�������ӿڵ�����˳��Ϊ��װ����ͼ1�����ظ�ʹ�ã�a �� ���������� ��
��3������Ʒ��ַ�Ӧ�����ͨ�������Ŀ���� ��
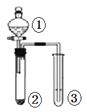
��4������ II�ٳ�ȡ��ʽ̼��þ��Ʒmg���ڽ���Ʒ��ָ������գ���ȴ����������ظ������ڣ����ʣ���������Ϊm1g ����������ͼ2�У��÷��������õ����� ��
��5���ж���Ʒ��ȫ�ֽ�ķ���������ʵ��������Ҫ�����Σ�
��6����ͬѧ��Ϊ���� II�������յĹ����лᷢ��4MgCO3+SiO2 ![]() MgSiO3+CO2���ᵼ�²ⶨ�����������Ϊ��λͬѧ�Ĺ۵���ȷ�������ȷ��������˵�����ɣ� ��
MgSiO3+CO2���ᵼ�²ⶨ�����������Ϊ��λͬѧ�Ĺ۵���ȷ�������ȷ��������˵�����ɣ� ��
���𰸡�
��1��4MgCO3?Mg��OH��2?5H2O+5H2SO4=5MgSO4+11H2O+4CO2��
��2��d��e��b��c��b��c����ȥ������̼�����е�ˮ����
��3����װ���в�����CO2ȫ���ϳ�����ȫ����
��4��E
��5����Ʒ�������θ�������,��ȴ�����������0.1g���ڣ�4
��6�����÷�Ӧ�ķ�����Ӱ������CO2��ˮ��������
���������⣺��1�����з�����Ӧ�Ǽ�ʽ̼��ͭ��ϡ���ᷢ�����ֽⷴӦ��������þ��ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��4MgCO3Mg��OH��25H2O+5H2SO4=5MgSO4+11H2O+4CO2����
���Դ��ǣ�4MgCO3Mg��OH��25H2O+5H2SO4=5MgSO4+11H2O+4CO2������2��ȡһ����������Ʒ���������ַ�Ӧ��ͨ���ⶨCO2���������㴿�ȣ���K����װ�ü���ͨ�������ͨ������������Һ���ն�����̼��ֹ���Ŷ�����̼�ⶨ���ɾ�װ���ڿ������ر�K��ͨ��װ���ҷ�Һ©������ϡ���ᷢ����Ӧ���ɶ�����̼��ˮ������þ��ͨ��װ�ö����������е�ˮ������ͨ��װ�ñ��������ɵĶ�����̼�ⶨ������Ϊ��������ж�����̼��ˮ�����������ܣ����������Ӹ���ܣ������ӿڵ�����˳��Ϊa��d��e��b��c��b��c�����������dz�ȥ������̼�����е�ˮ������
���Դ��ǣ�d��e��b��c��b��c����ȥ������̼�����е�ˮ��������3���ر�ֹˮ��K������Ʒ�м���������ϡ���ᣬ����Ʒ��ַ�Ӧ���Ϊ�˲ⶨȷ��Ӧ���еIJ����ǣ���ֹˮ��K�Ӽ���ͨ������������ɵĶ�����̼����ȫ�������������գ�
���Դ��ǣ���װ���в�����CO2ȫ���ϳ�����ȫ���գ���4��ȡ��ʽ̼��þ��Ʒmg���ڽ���Ʒ��ָ���ȼ�գ���ȴ����������ظ������ڣ����ʣ���������Ϊm1g����������ƽ�����������չ���ѡ�������ڼ������գ���Ҫ���żܡ������ǡ��ƾ��ơ��������ȣ�����Ӧ�ڸ������н��У����Բ���Ҫ����������
���Դ��ǣ�E����5���ж���Ʒ��ȫ�ֽ�ķ�������Ʒ�������θ������գ���ȴ�����������0.1g���ڣ����� II�ٳ�ȡ��ʽ̼��þ��Ʒmg���ڽ���Ʒ��ָ������գ���ȴ����������ظ������ڣ����ʣ���������Ϊm1g�������������0.1g���ڣ��������ٳ���4�Σ�
���Դ��ǣ���Ʒ�������θ������գ���ȴ�����������0.1g���ڣ�4����6������MgCO3+SiO2 ![]() MgSiO3+CO2�����÷�Ӧ�ķ�����Ӱ������CO2��ˮ������������λͬѧ�Ĺ۵��Ǵ���ģ�
MgSiO3+CO2�����÷�Ӧ�ķ�����Ӱ������CO2��ˮ������������λͬѧ�Ĺ۵��Ǵ���ģ�
���Դ��ǣ����÷�Ӧ�ķ�����Ӱ������CO2��ˮ����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺
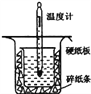
(1)����ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��___________________��
(2)������60mL 0.25 molL-1H2SO4��50mL 0.55molL-1NaOH��Һ���з�Ӧ������ʵ����ȣ����ų�������_____________(���ȡ�������ȡ�)����ʵ���������ȷ���������к���______(���ȡ�������ȡ�)
(3) д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ57.3kJ/mol)��_____________________________________��
(4)ijѧ��ʵ���¼�������£�
ʵ�� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
��� | ���� | ����������Һ | �����Һ |
1 | 20.0 | 20.1 | 23.2 |
2 | 20.2 | 20.4 | 23.4 |
3 | 20.5 | 20.6 | 23.6 |
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к��ȡ�H=__________(�������һλС��)��
(5)����ʵ����ֵ�����57.3kJ/mol ��ƫ�����ƫ���ԭ�������________________________��
a.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������¶�
b.��ȡ��������ʱ���Ӷ���
c.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d.ʵ��װ�ñ��¡�����Ч����
����Ŀ����̩����һ�ִ�ͳ���ֹ���Ʒ������������̩���IJ����У����漰��ѧ�仯���ǣ� ��
A | B | C | D |
��ͭ˿ѹ�⣬����ͼ�� | ��Ǧ���������εȻ������ƺ���� | ���±��� | ��ϴȥ�� |
A.A
B.B
C.C
D.D
����Ŀ��������ͼ��ʾװ�ý�����������ʵ�飬�����õ���Ӧʵ����۵���
ѡ�� | �� | �� | �� | ʵ����� |
|
A | Ũ���� | Na2SO3 | ����KMnO4��Һ��dz | SO2�л�ԭ�� | |
B | Ũ���� | KMnO4 | FeBr2��Һ��Ϊ��ɫ | �����ԣ�Cl2 >Br2 | |
C | ϡ���� | ̼���� | CaCl2��Һ�����Ա仯 | CO2����CaCl2��Ӧ | |
D | Ũ���� | ���� | ��ˮ��ɫ | Ũ���������ˮ�ԡ�ǿ������ |
A. A B. B C. C D. D