��Ŀ����
����Ŀ������������������������Ϳ��������Ź㷺��Ӧ�á�
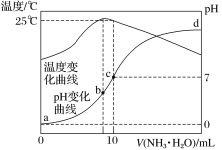
��1����ҵ�ϲ���NH3Ϊ��ԭ���������������������е�NO��NO2�����ʵ�����ȣ��ҷ�Ӧ����Ϊ�����壩�������ݳ������е������ﺬ�����Ӷ�ȷ�������ѵ��ʡ���ͼ��������˵����ȷ����(��д������ĸ���)

a����ͬ�����£��ı�ѹǿ���ѵ�����Ӱ��
b��������ߵ��ʾ��ʱƽ��ת�������
c����ͬ�����£����벻ͬ�Ĵ����ܹ��ı䷴Ӧ���ת����
d��������Ӧ�Ļ�ѧ����ʽΪ��NO��NO2��2NH3![]() 2N2
2N2![]() 3H2O
3H2O
��2����֪�� 2NH3(g)+CO2(g)![]() CO(NH2)2(s)+H2O(g) ��H=��43.0KJ��mol-1
CO(NH2)2(s)+H2O(g) ��H=��43.0KJ��mol-1
��������������2molNH3��1molCO2�����ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ����2minʱ���ų�����21.5kJ����2min�ڸ÷�Ӧ�ķ�Ӧ���ʦ�(NH3)= ����ʱ���������NH3���������Ϊ ��
��3����25���£���a molL-1��NH4NO3��Һ��0.01molL-1��NaOH�������ϣ���Ӧƽ��ʱ�������ҺpH=7����NH4NO3��Һ�����ʵ���Ũ��a___________0.01molL-1������������������������μ�NaOH�Ĺ�����ˮ�ĵ���ƽ�⽫ ����������������������ƶ����������ʵ�����Ƚ�NH3 H2O�ĵ���̶Ⱥ�NH4NO3��ˮ��̶ȴ�С ��
���𰸡���1��ad
��2��0.25molL-1min-1��50%����0.5��
��3�����������������£��������ʵ���Ũ�ȡ�������İ�ˮ���������Һ��ϣ������ҺpH>7����������̶ȴ���ˮ��̶ȣ��������̶�С��ˮ��̶ȣ�������������ɵ÷֣���
��������
�����������1��a����Ϊ�÷�Ӧǰ���������������ȣ����Ըı�ѹǿƽ���ƶ����ѵ��ʱ仯����a��ȷ��b�����������¶ȣ�����������ת����һ��������Ӧ�õ�һ�������������������߱仯���˵���÷�Ӧ��ϵ���д������ڣ�����ߵ���������������b������c������ֻ��Ӱ�컯ѧ��Ӧ���ʣ����ܸı仯ѧƽ��״̬�����ԣ���ͬ�����£����벻ͬ�Ĵ������ܸı䷴Ӧ���ת��������c������d������NH3Ϊ��ԭ�������������������е�NO��NO2�����ʵ�����ȣ��ҷ�Ӧ����Ϊ����������NH3Ϊ��ԭ����NO��NO2Ϊ���������ҷ�Ӧ����ΪN2����Ӧ�Ļ�ѧ����ʽΪ��NO��NO2��2NH3![]() 2N2+3H2O����d��ȷ��
2N2+3H2O����d��ȷ��
��2���ָ�����������μӷ�Ӧ�İ�������n(NH3)=21.5kJ��43.0 kJ/mol��2=1mol����c(NH3)=1mol/2L=0.5mol/L���ٸ��ݷ�Ӧ���ʹ�ʽ����v(NH3)=��c(NH3)/��t=0.5mol/L��2min=0.25molL-1min-1����������ʽ2NH3 +CO2 CO(NH2)2(s)+H2O(g)��֪������1mol�����μӷ�Ӧʱ��������������1mol����ʱ���������NH3���������Ϊ��(2-1)/(2+1-1)��100%=50%����0.5����
��3����25���£�����NH4NO3��Һˮ���̶Ⱥ�С���ʼ��������ԣ�����0.01molL-1��NaOH�������ϣ������ҺpH=7ʱ��NH4NO3��Һ�����ʵ���Ũ��a��0.01molL-1��NH4NO3ˮ���ٽ�ˮ�ĵ��룬�μ�NaOH�Ĺ�����笠�����Ũ����С��ˮ��̶ȼ�С��ˮ�ĵ���ƽ�⽫�����ƶ������Ƚ�NH3H2O�ĵ���̶Ⱥ�NH4NO3��ˮ��̶ȴ�С�������������£��������ʵ���Ũ�ȡ�������İ�ˮ���������Һ��ϣ������ҺpH>7����������̶ȴ���ˮ��̶ȣ��������̶�С��ˮ��̶ȣ�������������ɵ÷֣�������Ϊ�����������������£��������ʵ���Ũ�ȡ�������İ�ˮ���������Һ��ϣ������ҺpH>7����������̶ȴ���ˮ��̶ȣ��������̶�С��ˮ��̶ȣ�������������ɵ÷֣���