题目内容
(9分)某化学课外兴趣小组为探究苯与溴发生反应的反应类型并制取纯净的溴苯,进行如下实验。请根据要求回答相关问题。
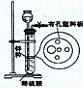
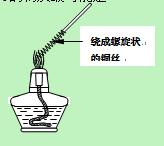
I:制取溴苯(装置如图所示)

(1)向烧瓶中滴人溴的苯溶液片刻后,在烧瓶中可观察到的现象是
(2)锥形瓶中有淡黄色浑浊生成,A同学提出不能由此证明苯与溴发生了取代反应,原因是 。
(3)A同学从下列装置中选取适宜装置进行改进,请帮A同学在下面实验流程图的方框中填入序号。
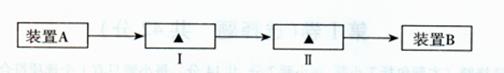
①装有NaOH溶液的洗气瓶 ②装有CC14的洗气瓶
③装有KI溶液的洗气瓶 ④装有湿润淀粉KI试纸的集气瓶
(4)该实验还可用 代替AgNO3溶液检验取代反应的产物之一溴化氢。
Ⅱ:提纯溴苯
(5)反应后所得混合液,经以下几步操作可得纯净的溴苯。简要写出各操作目的(不一定填满)。
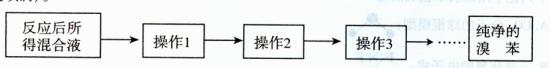
操作l目的: 。操作2目的: 。
。操作2目的: 。
操作3目的: 。操作4目的: 。
I:制取溴苯(装置如图所示)

(1)向烧瓶中滴人溴的苯溶液片刻后,在烧瓶中可观察到的现象是
(2)锥形瓶中有淡黄色浑浊生成,A同学提出不能由此证明苯与溴发生了取代反应,原因是 。
(3)A同学从下列装置中选取适宜装置进行改进,请帮A同学在下面实验流程图的方框中填入序号。

①装有NaOH溶液的洗气瓶 ②装有CC14的洗气瓶
③装有KI溶液的洗气瓶 ④装有湿润淀粉KI试纸的集气瓶
(4)该实验还可用 代替AgNO3溶液检验取代反应的产物之一溴化氢。
Ⅱ:提纯溴苯
(5)反应后所得混合液,经以下几步操作可得纯净的溴苯。简要写出各操作目的(不一定填满)。

操作l目的:
 。操作2目的: 。
。操作2目的: 。操作3目的: 。操作4目的: 。
(12分)
(1)ABC(1分) 会加大副反应进行( 2分)
2分)
(2)Ⅱ可以防止挥发性尾气进入空气污染环境(2分) B(1分)
(3)①③④⑥(2分)
(4)从任意一层取少量溶液加水,如混溶液则该层为水层;不混溶则为有机层(其他合理答案给分) (2 分)(5)测定产品的沸点(其他合理答案给分)(2分)
分)(5)测定产品的沸点(其他合理答案给分)(2分)
(1)ABC(1分) 会加大副反应进行(
 2分)
2分) (2)Ⅱ可以防止挥发性尾气进入空气污染环境(2分) B(1分)
(3)①③④⑥(2分)
(4)从任意一层取少量溶液加水,如混溶液则该层为水层;不混溶则为有机层(其他合理答案给分) (2
 分)(5)测定产品的沸点(其他合理答案给分)(2分)
分)(5)测定产品的沸点(其他合理答案给分)(2分)略

练习册系列答案
相关题目

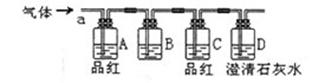
 2NH3(g)+CO2(g)
2NH3(g)+CO2(g)
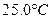 时,0-6min 氨基甲酸铵水解反应的平抑速率 ______。
时,0-6min 氨基甲酸铵水解反应的平抑速率 ______。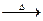 CH3COOH+Cu2O↓+2H2O”提出了质疑,并进行如下探究:
CH3COOH+Cu2O↓+2H2O”提出了质疑,并进行如下探究: