��Ŀ����
20��Ԫ�ظ����仯���﹤ҵ��;�㷺������+6�۸�����ˮ����������Ƴ������Ķ�ͭ��ˮ����������һ������Cr2O32-�������÷�ˮ����ֱ�ӳ�������ԭ��������I��ֱ�ӳ�����
��1����֪������ˮ�д�����ƽ�⣺Cr2O32-+H2O?2CrO42-+2H+����ʵ�ʹ�ҵ�����У����������BaCl2��Һ֮ǰ��Ҫ����һ������NaOH�����������ڳ��������ɣ������ɳ����Ļ�ѧʽΪBaCrO4
��ԭ������
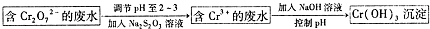
��2��������Һ�п��Դ�������������Na2S2O3��Һ����AD����ѡ����ţ���
A��FeSO4��ҺB��ŨH2SO4C������KMnO4��ҺD��Na2SO3��Һ
��3�����������У�ÿ����0.1molNa2S2O3ת��0.8mole-�������Na2S2O3��Һʱ������Ӧ�����ӷ���ʽΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O
��4��Cr��OH��3�Ļ�ѧ������Al��OH��3���ƣ����������������м���NaOH��ҺʱҪ������Һ��pH���ܹ��ߣ�ԭ��������ӷ���ʽ��ʾ��Cr��OH��3+OH-=CrO2-+2H2O
��5��ʵ�ʹ�ҵ��������ʱ�����������ӽ�����֬�����ⶨ��������Һ��Cr3+�ĺ�������ԭ����Mn++nNa++MRn������NaHΪ�����ӽ�����֬��Mn+ΪҪ�ⶨ�����ӣ�
�������ӽ�����֬��ԭ����֮һ�Ǿ۱���ϩ���䵥��Ϊ����ϩ����
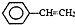 �������۱���ϩ�Ļ�ѧʽΪ
�������۱���ϩ�Ļ�ѧʽΪ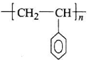
��ij�βⶨ�����У�pH=5�ķ�ˮ���������ӽ�����֬�����Һ��Na+�Ƚ���ǰ������4.6��10-2g•L-1�����������Cr��OH��3��K�յ�ֵΪ6.7��10-31��
���� ��1�����������Ӻ������ӷ�Ӧ����ˮ����ʹƽ��Cr2O72-+H2O?2CrO42-+2H+���ƣ������ڸ�Ԫ��ת��Ϊ���ᱵ��BaCrO4��������
��Cr2O72-���ӵķ�ˮ����Na2S2O3��Һ������ҺPH=2-3����ԭ�ظ�������ӵõ�Cr3+���ӵ���Һ����������������Һ������ҺPH����Cr��OH��3��
��2�����Դ�������������Na2S2O3��Һ����Ҫ���л�ԭ�ԣ��ܻ�ԭ�ظ�������ӣ�
��3��ÿ����0.1mol Na2S2O3ת��0.8mol e-��Na2S2O3 ��2SO42-��8e-��Cr2O72-��2Cr3+��6e-������������ԭ��Ӧ�����غ������ƽ��д������ԭ��Ӧ�����ӷ���ʽ��
��4��Cr��OH��3�Ļ�ѧ������Al��OH��3���ƣ���ʾ���ԣ������ܽ���ǿ�ᡢǿ����Һ�У�
��5���پ۱���ϩ�DZ���ϩһ�������·����ļӳɾۺϷ�Ӧ�������˸߷��ӻ�����۱���ϩ��
�������ӽ�����֬�����ⶨ��������Һ��Cr3+�ĺ�������ԭ����Mn++nNaR�TnNa++MRn������NaRΪ�����ӽ�����֬��Mn+ΪҪ�ⶨ�����ӣ����㱻����������Cr3+Ũ�ȣ���pH=5�ķ�ˮ������������Ũ��Ϊ10-9mol/L������ܶȻ������������Ksp��
��� �⣺��1��������ˮ�д�����ƽ�⣺Cr2O72-+H2O?2CrO42-+2H+����ʵ�ʹ�ҵ�����У����������BaCl2��Һ֮ǰ��Ҫ����һ������NaOH�����������ڳ��������ɣ����������Ӻ������ӷ�Ӧ����ˮ����ʹƽ��������У������ڸ�Ԫ��ת��Ϊ���ᱵ�����������ɳ����Ļ�ѧʽΪ��BaCrO4��
�ʴ�Ϊ��BaCrO4��
��2�����Դ�������������Na2S2O3��Һ����Ҫ���л�ԭ�ԣ��ܻ�ԭ�ظ�������ӣ�
A��FeSO4��Һ���������Ӿ��л�ԭ�ԣ����Ի�ԭCr2O72-���ӣ���A���ϣ�
B��ŨH2SO4 ����ǿ�����ԣ����ܱ��ֻ�ԭ�ԣ����ܻ�ԭCr2O72-����B�����ϣ�
C������KMnO4 ��ǿ���������ܻ�ԭCr2O72-����C�����ϣ�
D��Na2SO3��Һ������������Ӿ��л�ԭ�ԣ����Ի�ԭCr2O72-����D���ϣ�
�ʴ�Ϊ��AD��
��3��ÿ����0.1mol Na2S2O3ת��0.8mol e-��Na2S2O3 ��2SO42-��8e-��Cr2O72-��2Cr3+��6e-������������ԭ��Ӧ�����غ���ƽ��д��3Na2S2O3 ��6SO42-��24e-��4Cr2O72-��8Cr3+��24e-���õ���������ԭ��Ӧ�����ӷ���ʽΪ��3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
�ʴ�Ϊ��3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
��4�����������������м���NaOH��ҺʱҪ������Һ��PH���ܹ��ߣ�Cr��OH��3�Ļ�ѧ������Al��OH��3���ƣ�Cr��OH��3�����ܽ��ڹ���������������Һ�У��������ӷ���ʽ��ʾԭ��Ϊ��Cr��OH��3+OH-=CrO2-+2H2O��
�ʴ�Ϊ��Cr��OH��3+OH-=CrO2-+2H2O��
����5���پ۱���ϩ�DZ���ϩһ�������·����ļӳɾۺϷ�Ӧ�������˸߷��ӻ�����۱���ϩ���䵥��Ϊ����ϩ��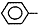 CH=CH2������ӦΪ��n
CH=CH2������ӦΪ��n CH=CH2��
CH=CH2�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
�������ӽ�����֬�����ⶨ��������Һ��Cr3+�ĺ�������ԭ����Cr3++3NaR�T3Na++CrR3������NaRΪ�����ӽ�����֬��Mn+ΪҪ�ⶨ������Cr3+��Na+�Ƚ���ǰ������4.6��10-2g•L-1�����ʵ���Ũ��=$\frac{4.6}{23}��1{0}^{-2}$mol/L=2��10-3mol/L�����㱻����������Cr3+Ũ��=$\frac{1}{3}$��2��10-3mol/L��Cr��OH��3��s��?3c��OH-��+c��Cr3+������pH=5�ķ�ˮ���������ӽ�����֬����Һ��c��OH-��=10-9mol/L��Ksp=c3��OH-��c��Cr3+��=[10-9]3��$\frac{1}{3}$��2��10-3mol/L=6.7��10-31��
�ʴ�Ϊ��6.7��10-31��
���� ���⿼�������ʷ����ᴿ�ķ������̷��������ӷ���ʽ��д���ܶȻ������ļ���Ӧ�ã���Ҫ��������ԭ��Ӧ������Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

| A�� | ������ͬ���칹�� | B�� | �ǷǼ��Է��� | ||
| C�� | ֻ��һ�ֽṹ����ͬ���칹�� | D�� | ��һ������� |
| A�� | ��������IVA�� | B�� | ��������IVA�� | C�� | ��������VA�� | D�� | ��������VA�� |
 Ϊ�˽��������ȾΣ�����й�ú̿ҵ�ƶ��ˡ�ú�������ƻ�����ҵ�Ͽ���ú�����ϳ�����CO��H2����Ҳ����ú����Ȼ����
Ϊ�˽��������ȾΣ�����й�ú̿ҵ�ƶ��ˡ�ú�������ƻ�����ҵ�Ͽ���ú�����ϳ�����CO��H2����Ҳ����ú����Ȼ������1����֪����CO��g��+H2O��g���TH2��g��+CO2��g����H=-41kJ•mol-1
��C��s��+2H2��g���TCH4��g����H=-73kJ•mol-1
��2CO��g���TC��s��+CO2��g����H=-171kJ•mol-1
д��CO2��H2��Ӧ����CH4��H2O���Ȼ�ѧ����ʽ��CO2��g��+4H2��g��=CH4��g��+2H2O��g����H=-162 kJ•mol-1��
��2�����úϳ������Ʊ�����ȼ�ϼ״���CO��g��+2H2��g��?CH3OH��g����H���±����������Ǹ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
| �¶� | 250�� | 300�� | 350�� |
| K | 2.041 | 0.270 | 0.012 |
��ij�¶��£���2mol CO��6mol H2����2L�ĺ����ܱ������У���ַ�Ӧ���ﵽƽ����c��CO��=0.2mol/L����CO��ת����Ϊ80%����ʱ���¶�Ϊ250�棨���ϱ���ѡ��
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�ã�����һ��ʱ������Һ��pH��С����õ���ܷ�Ӧ�����ӷ���ʽΪ2CH3OH+3O2+4OH-�T2CO32-+6H2O��
| A�� | 4.48L | B�� | 5.6L | C�� | 6.72L | D�� | 13.44L |
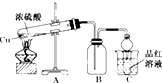 ij��ѧ��ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾװ�ý����й�ʵ�飮
ij��ѧ��ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾװ�ý����й�ʵ�飮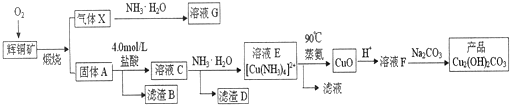
 �л���Ӧ�г�������������ij�������к�Ni 64.0%��Al 24.3%��Fe 1.4%������ΪC��H��O��N��Ԫ�أ�
�л���Ӧ�г�������������ij�������к�Ni 64.0%��Al 24.3%��Fe 1.4%������ΪC��H��O��N��Ԫ�أ�