��Ŀ����
�ҹ��зḻ�ĺ�ˮ��Դ�����������ú�ˮ��Դ�ǵ�ǰ��ѧ�о���һ����Ҫ������ͼ��ij�������Ժ�ˮ��Դ�ۺ����õ�ʾ��ͼ
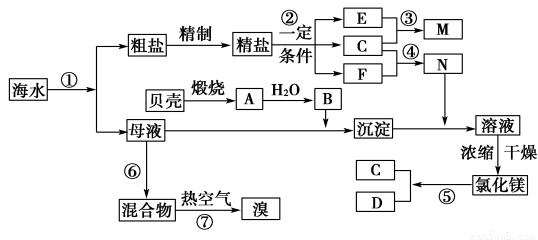
�����������Ϣ�ش��������⣺
(1)��ͼ�ܵķ�Ӧ�����ǵ�ȼ����д��N_________���ѧʽ����
(2)д����Ӧ�ڵ�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ��______________________________��
��3�������к���Ca2����Mg2����SO�����ʣ�����ʱ���õ��Լ�Ϊ�������� ���Ȼ�����Һ ������������Һ ��̼������Һ�������Լ����ӵ�˳�����Ϊ____________��
A���ڢۢܢ� B���ۢڢܢ�
C���ܢۢڢ� D���ۢܢڢ�
��4����ȡ���κ�ʣ��ĺ�ˮ(ĸҺ)�У���������ȡMg������������ȡMg�����̣�û���漰���ķ�Ӧ������____________��
A���ֽⷴӦ B�����Ϸ�Ӧ C�����ֽⷴӦ D���û���Ӧ
��5����ȡ���κ�ʣ��ĺ�ˮ(ĸҺ)�У���������ȡBr2����Ӧ�����õ���̬��������Ѱ�һ�Դ�����룬���к�������____________��
A������ع��� B���ڵ����½�������
C���ӱ��������ռѭ�� D���ӱ�������þ���ʴ�ѭ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д����г����µ�������Һ(���±�)�������й�������ȷ����( )
�� | �� | �� | �� | |
��ˮ | �������� | ���� | ���� | |
pH | 11 | 11 | 3 | 3 |
A���ֱ��ˮϡ��10����������Һ��pHΪ����>��>��>��
B���¶�����10�棬������Һ��pH����
C���ۢ��зֱ�����������Ȼ�茶���۵�pH��С���ܵ�pH����
D�����٢�������Һ�������ϣ�������Һ��c (C1-) >c NH4+)>c (H+)>c (OH- )
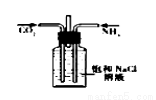
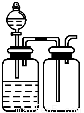
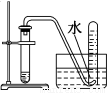
����ѡ�õ�ʵ��������ʵ��װ�÷���ʵ��Ҫ���Ұ�ȫ����( )
A | B | C | D |
��NaCl��Һ��ͨNH3����ͨCO2�Ʊ�NaHCO3 | ʵ�����Ʊ��������� | ʵ�����Ʊ�Cl2 | ����O2��� |
|
|
|
|



 ��ͬ����ķ��������������ԭ�������
��ͬ����ķ��������������ԭ������� 2SO3��g�������ﵽƽ�⡣��������У����������ֺ��º��ݣ����������ֺ��º�ѹ���ﵽƽ��ʱ������˵����ȷ���ǣ� ��
2SO3��g�������ﵽƽ�⡣��������У����������ֺ��º��ݣ����������ֺ��º�ѹ���ﵽƽ��ʱ������˵����ȷ���ǣ� ��