��Ŀ����
��֪W��X��Y��Z��ԭ��������������Ķ�����Ԫ�أ�W��X ��Z���Ƿǽ���Ԫ�أ������µ��ʶ�Ϊ��̬��X��Z��ͬһ���壬 Y����Ϊ����ɫ�������壬��W��Y��������������ȣ�Y��Zͬ�����һ�����YZ�Ǻ�ˮ��Ҫ�ijɷ�֮һ��
��1��W��������ɵ������W���ʺ�O2���÷�Ӧ��ת�Ƶĵ�������Ϊ ��
��2��X2����ˮ��Ӧ����WX��O2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��Y����ˮ��Ӧ����YOH��W2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��Z2�ǻ���ɫ�д̼�����ζ�����壬����ˮ��Ӧ����HZ��HZO��
�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5���Ƚ�HX��HZ��̬�⻯����ȶ��ԣ� > ���û�ѧʽ��ʾ��
��6��YOH��һ��ǿ�0.25 mol�ĸ�������һ����HZϡ��Һ�������ԣ������кͷ�
Ӧ�����ų� Q kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��
��1��W��������ɵ������W���ʺ�O2���÷�Ӧ��ת�Ƶĵ�������Ϊ ��
��2��X2����ˮ��Ӧ����WX��O2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��Y����ˮ��Ӧ����YOH��W2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��Z2�ǻ���ɫ�д̼�����ζ�����壬����ˮ��Ӧ����HZ��HZO��
�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5���Ƚ�HX��HZ��̬�⻯����ȶ��ԣ� > ���û�ѧʽ��ʾ��
��6��YOH��һ��ǿ�0.25 mol�ĸ�������һ����HZϡ��Һ�������ԣ������кͷ�
Ӧ�����ų� Q kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��
��1��4
��2��2F2 + 2H2O =" 4HF" + O2��
��3��2Na + 2H2O =" 2NaOH" + H2��
��4��Cl2 + H2O =" HCl" + HclO
��5��HF �� HCl
��6��NaOH + HCl =" NaCl" + H2O + 4Q KJ
��2��2F2 + 2H2O =" 4HF" + O2��
��3��2Na + 2H2O =" 2NaOH" + H2��
��4��Cl2 + H2O =" HCl" + HclO
��5��HF �� HCl
��6��NaOH + HCl =" NaCl" + H2O + 4Q KJ
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


 .074 n m
.074 n m 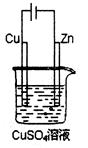
 ��128g����Yһ����ȼ�ϵ�����������ı�״���µĿ�
��128g����Yһ����ȼ�ϵ�����������ı�״���µĿ�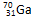 ��
��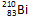 ��ԭ�Ӿ��˾ۺϣ����ų�һ����Ŀ�����Ӷ��Ƶ�������Ϊ275��������Ϊ114��ijԭ�ӡ�����ԭ���ں˾ۺϹ����зų���������Ŀ��
��ԭ�Ӿ��˾ۺϣ����ų�һ����Ŀ�����Ӷ��Ƶ�������Ϊ275��������Ϊ114��ijԭ�ӡ�����ԭ���ں˾ۺϹ����зų���������Ŀ��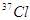 ����_____�����ӣ�_____�����ӣ�_____�����ӡ���������������_____���ȵ���������ﻯѧʽΪ__________����̬�⻯��Ļ�ѧʽΪ__________��
����_____�����ӣ�_____�����ӣ�_____�����ӡ���������������_____���ȵ���������ﻯѧʽΪ__________����̬�⻯��Ļ�ѧʽΪ__________�� ��������ͣ���
��������ͣ���