��Ŀ����
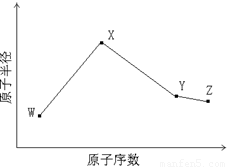
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ14��������Ϊ7��X��������NH4+������ͬ�����ӡ�������Ŀ�� W��Y����������ܵ���������γɣ�Z�ķǽ�������ͬ��������Ԫ������ǿ��
��1��Y�����ڱ��е�λ���� ��
��2���õ���ʽ����X��W���γɻ�����X3W��ԭ�� ��
��3��X3W��ˮ���ͷų�ʹ��̪��Һ��������A����ѧ����ʽ�� ��
��4���ö��Ե缫��⻯����XZ��Һ�������ͷų�����B����Ӧ�����ӷ���ʽ�� ��
��5����֪W�ĵ���������B��һ�������¿��γ�����A������ W2 (g)+3B (g) 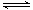 2A(g)
��H =��92.4 kJ・mol�D1
2A(g)
��H =��92.4 kJ・mol�D1
��ij�¶�ʱ��һ���ݻ��̶����ܱ������У�����������Ӧ���ڲ�ͬʱ��ⶨ�������ڸ����ʵ�Ũ�����±���
|
ʱ�� |
Ũ��(mol/L) |
||
|
c(W2) |
c(B) |
c(A) |
|
|
��0 min |
4.0 |
9.0 |
0 |
|
��10min |
3.8 |
8.4 |
0.4 |
|
��20min |
3.4 |
7.2 |
1.2 |
|
��30min |
3.4 |
7.2 |
1.2 |
|
��40min |
3.6 |
7.8 |
0.8 |
��W2��ƽ����Ӧ����v(0min��10min)/ v(10min��20min) = ��
�ڷ�Ӧ�ڵ�10min�ı��˷�Ӧ�������ı������������ ��
a�������˴��� b�������¶� c������ѹǿ d������B��Ũ��
������Ӧ�ӵ�30minĩ�ַ�����һ�������ı䣬�ı�ķ�Ӧ���������� ��
a�������˴��� b�������¶� c������ѹǿ d����СA ��Ũ��
��1�� �������ڡ��ڢ����� ��2��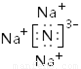
��3��Na3N+3H2O =NH3��+3NaOH
��4��2Cl-+2H2O  Cl2��+H2��+2OH-��5���� 1/2
�� a��b �� b
Cl2��+H2��+2OH-��5���� 1/2
�� a��b �� b
��������
�������������������=������-�������������ƶ�W��N��X��������NH4+������ͬ�����ӡ�������Ŀ��˵��X��Na��SO2���γ����꣬����Y��S��Z��ԭ��������S�ǽ����Ը�ǿ��Z��Cl��Nλ�����ڱ��е������ڡ��ڢ����壬Na������ǿ����N�γ����ӻ�����Na3N��Na3N��ˮ����ˮ�����ɰ�����NaOH�����NaCl��Һ�ȼҵ��ԭ����ע����������5������1:3��Ӧ�����ƶϳ��ǹ�ҵ�ϳɰ�����3H2+N2  2NH3��v(0min��10min)/ v(10min��20min) =
2NH3��v(0min��10min)/ v(10min��20min) =  =
=
 ����10-20min��Ӧ�������ӣ����Կ��Լӿ����ʵ�������a b d����d�ı�B��Ũ�ȣ������ݿɿ���B��Ũ��δ���ӣ������ų�d����30min���ı�������ƽ�������ƶ��������¶ȣ�ƽ�������ȷ�Ӧ�����ƶ���
����10-20min��Ӧ�������ӣ����Կ��Լӿ����ʵ�������a b d����d�ı�B��Ũ�ȣ������ݿɿ���B��Ũ��δ���ӣ������ų�d����30min���ı�������ƽ�������ƶ��������¶ȣ�ƽ�������ȷ�Ӧ�����ƶ���
���㣺����Ԫ�������ɡ���ѧ��Ӧԭ��

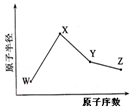 W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z��
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z��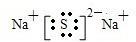
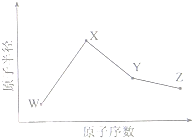 ��2011?���գ�W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ĵ縺����ͬ��������Ԫ�������
��2011?���գ�W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ĵ縺����ͬ��������Ԫ�������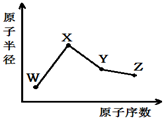 ��2013?����һģ��W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵����ȷ���ǣ�������
��2013?����һģ��W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵����ȷ���ǣ�������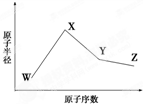 W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ĵ縺����ͬ��������Ԫ����������´𰸵�W��X��Y��Z��������Ӧ��Ԫ�ط��Ŵ��棩
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ĵ縺����ͬ��������Ԫ����������´𰸵�W��X��Y��Z��������Ӧ��Ԫ�ط��Ŵ��棩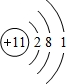
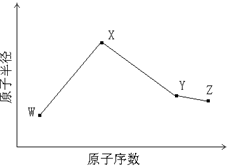 W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ14��������Ϊ7��X��������NH4+������ͬ�����ӡ�������Ŀ�� W��Y����������ܵ���������γɣ�Z�ķǽ�������ͬ��������Ԫ������ǿ��
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ14��������Ϊ7��X��������NH4+������ͬ�����ӡ�������Ŀ�� W��Y����������ܵ���������γɣ�Z�ķǽ�������ͬ��������Ԫ������ǿ��