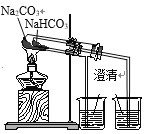
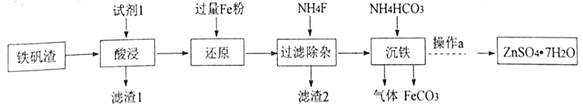
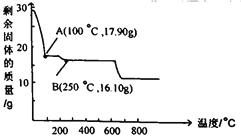
��Ŀ����
����Ŀ��ij����Na2O���ʵ�Na2O2������һ����ѧʵ��С������H2O��Na2O2�ķ�Ӧ���ⶨ����Ʒ�Ĵ��ȡ��ɹ�ѡ���װ�����£�

��ش��������⣺
��1��װ�â�������a��������______��
��2��������װ�ÿ�����װһ����IJⶨ����������Ʒ���ȵ�ʵ��װ�á�
��.��ʵ��װ�õ������________(����ĸ)��
a. �٢ܢ� b. �٢ۢ� c. �ڢܢ� d. �ۢݢ�
��.��ѡ��װ�õ�����˳��Ӧ��__________(����ӿڵ���ĸ�����ӽ���ʡ��)��
��3��д��ʵ����Na2O2������Ӧ�Ļ�ѧ����ʽ_____________��
��4��������ʵ���������Һ���Ƴ�Ũ��Ϊ1.0mol/L����Һ���ش��������⡣
����400mL����Һ��ͨ��0.3mol CO2����������Һ��HCO3�C��CO32�C�����ʵ���Ũ��֮��ԼΪ___________��
A. 1:3���� B. 1:2������C. 2:1��������D. 3:1
����������Һ�����ᾧ�õ�Na2CO3��NaHCO3�����������ѡ���в���ȷ�ⶨ�������Na2CO3������������____________��
a. ȡa g������ּ��ȣ��ڸ���������ȴ�����£�����b g
b. ȡa g�����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b g����
c. ȡa g�����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����b g
d. ȡa g�����������Ba(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b g����
���𰸡���Һ©�� A G��A��B��F 2Na2O2+2H2O=4NaOH+O2�� C c
��������
��װ����������a�������Ƿ�Һ©����
��������Ϊ��Ӧװ�ã�ͨ���ų�����ˮ������������еõ�ˮ������������������ƴ��ȣ�
��ʵ����Na2O2������Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2����
��HCO3�C��CO32�C�����ʵ����ֱ�Ϊx��y���������Ĺ�ϵ���м��㡣
��װ����������a�������Ƿ�Һ©����
��������Ϊ��Ӧװ�ã�ͨ���ų�����ˮ������������еõ�ˮ����������װ���DZȽϼIJⶨ����������Ʒ���ȵ�ʵ��װ�ã����A��ȷ��ѡ��װ�õ�����˳��Ӧ��G��A��B��F��
��ʵ����Na2O2������Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2����
����HCO3�C��CO32�C�����ʵ����ֱ�Ϊx��y����400mL����Һ�����ʵ���n=1.0mol/L��0.4L=0.4mol��ͨ��0.3mol CO2��x+y=0.3��x+2y=0.4�����x=0.2mol��y=0.1mol��Ũ��֮�ȵ������ʵ���֮�ȣ����������Һ��HCO3�C��CO32�C�����ʵ���Ũ��֮��ԼΪ2:1��C��ȷ��
��aѡ�ȡa g������ּ��ȣ��ڸ���������ȴ�����£�����b g�����ٵ�����Ϊ̼�����Ʒֽ����ɵĶ�����̼��ˮ������������ܼ����̼���Ƶ�������������a���������⣻
bѡ�ȡa g�����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b g���壬���õ��Ȼ��ƹ��壬���ݷ����������̼���Ƶ�������������b���������⣻
cѡ�ȡa g�����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����b g����ʯ�����ӵ������Ƕ�����̼��ˮ����������ˮ�����������㣬�ʲ��ܼ���̼���Ƶ�������������c�������⣻
dѡ�ȡa g�����������Ba(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b g���壬�õ�̼�ᱵ���������ݷ����������̼���Ƶ�������������d���������⣻
�����������𰸰�Ϊc��

����Ŀ����֪ij�Ͻ��ĩ�������⣬����������ͭ�е�һ�ֻ����֣�ij��ȤС������ʦ��ָ���£��ԺϽ�������ͭ�Ĵ����������������̽����
���������ϣ�����ͭ��������������Һ��Ӧ��
�����룩����1���úϽ��ĩ�У��������⣬����������
����2���úϽ��ĩ�У��������⣬������ͭ��
����3���úϽ��ĩ�У��������⣬������_________�������ƣ���
��ʵ��̽��������ʵ�����ѡ����Լ��ǣ�10%�����ᡢ30%������������Һ��
ʵ�鷽�� | ʵ������ | ���� |
��ȡһ�����ĺϽ��ĩ���ӹ�����____����ַ�Ӧ����ˣ��������á� | ��ĩ�����ܽ⣬��������ų��� | �Ͻ���һ���������� |
��ȡ����������������ӹ�����______����ַ�Ӧ�� | ������ĩ�����ܽ⣬��������ų�����Һ����dz��ɫ�� | �Ͻ���һ������______�� |
��̽�����ۣ�����3������
����˼��һ����˵�����ý�������������ᷴӦ���������ᡢ��ܷ�Ӧ��˵����������������ʡ�д����������������Һ��Ӧ�����ӷ���ʽ__________��
��֪ʶ���죩��һ�������£�������������ˮ��Ӧ��д������Ӧ�����£�����ˮ������Ӧ�Ļ�ѧ����ʽ_______________��