��Ŀ����
����Ŀ��һЩ�����ڹ�ҵ����������;���������Ϊԭ�Ͽ����Ƶ����ἰ��������ش�����������
��1������(H3BO3)ΪһԪ�ᣬ������B�Ļ��ϼ�Ϊ__________________��
��2����֪��Ka(H3BO3)=5.8��10-l0��Ka(H2CO3)=4.4��10-7��Ka(HCO3-)=4.7��10-l1����������Һ�еμ�0.1 molL-1Na2CO3��Һ��____________�����������������������۲쵽�����ݳ���д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________��
��3��������Ϊԭ�Ͽ��Ʊ���Ҫ��ԭ��NaBH4��BH4-�ĵ���ʽΪ______________��NaBH4��BF3��50��~70����Ӧ����NaBF4��������(B2H6)���÷�Ӧ�Ļ�ѧ����ʽ��___________________________��
��4�������ܽ��������Ƶú�Fe3+��Fe2+��Al3+���ʵ�������Һ���ᴿ�����м���H2O2��Ŀ����____________________��Ϊ��ȥFe3+��Al3+ (ʹ��Ũ�Ⱦ�С��1��10-6 mol��L-1)�������ٵ���pH=_________����֪�� Ksp[Al(OH)3]= 1��10-33��Ksp[Fe(OH)3]=4��10-38��
��5��H3BO3����ͨ�����ķ����Ʊ����乤��ԭ����ͼ��ʾ����Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ������
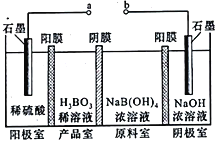
��a�ӵ�Դ��____________ ���������������� ��������
��д�������ĵ缫��Ӧʽ__________________________________��
��ԭ���������������������Ǩ�Ƶģ�_______________________________________��
���𰸡� +3 ���� NaCO3+H3BO3=NaHCO3+NaH2BO3 ![]() 3NaBH4+4BF3
3NaBH4+4BF3![]() 3NaBF4+2B2H6 ��Fe2+��������Fe3+�����ڳ�ȥ 5 ���� 2H2O-4e-=O2��+4H+ [B(OH)4]-������Ĥ�����Ʒ�ң�Na+������Ĥ����������
3NaBF4+2B2H6 ��Fe2+��������Fe3+�����ڳ�ȥ 5 ���� 2H2O-4e-=O2��+4H+ [B(OH)4]-������Ĥ�����Ʒ�ң�Na+������Ĥ����������
����������1������(H3BO3)��B�Ļ��ϼ�Ϊ+3����2������Ka(H3BO3)=5.8��10-l0����̼���һ������������֮�䣬��������Һ�еμ�0.1 molL-1Na2CO3��Һ������̼�����ƣ��۲첻�������ݳ�����Ӧ�Ļ�ѧ����ʽΪ��NaCO3+H3BO3=NaHCO3+NaH2BO3����3��BH4-�ĵ���ʽΪ![]() ��NaBH4��BF3��50��~70�淴Ӧ����NaBF4��������(B2H6)����Ӧ�Ļ�ѧ����ʽ��3NaBH4+4BF3
��NaBH4��BF3��50��~70�淴Ӧ����NaBF4��������(B2H6)����Ӧ�Ļ�ѧ����ʽ��3NaBH4+4BF3![]() 3NaBF4+2B2H6����4������H2O2��Ŀ���ǽ�Fe2+��������Fe3+�����ڳ�ȥ����ΪKsp[Al(OH)3]= 1��10-33����Ksp[Fe(OH)3]=4��10-38��ֻҪ��ȥAl3+ (ʹ��Ũ�Ⱦ�С��1��10-6 mol��L-1)��Fe3+�϶��������ˣ���Ksp[Al(OH)3]= 1��10-33��c(Al3+)=1��10-6 mol��L-1����Ksp[Al(OH)3]����ʽ�����c(OH-)=1��10-9��pH=5����5����ͼ�����������������������֪����a�ӵ�Դ���������������ĵ缫��ӦʽΪ��2H2O-4e-=O2��+4H+ ���۴Ӳ�Ʒ���ɽǶȷ�����֪��ԭ������[B(OH)4]-������Ĥ�����Ʒ�ң�Na+������Ĥ���������ҡ�
3NaBF4+2B2H6����4������H2O2��Ŀ���ǽ�Fe2+��������Fe3+�����ڳ�ȥ����ΪKsp[Al(OH)3]= 1��10-33����Ksp[Fe(OH)3]=4��10-38��ֻҪ��ȥAl3+ (ʹ��Ũ�Ⱦ�С��1��10-6 mol��L-1)��Fe3+�϶��������ˣ���Ksp[Al(OH)3]= 1��10-33��c(Al3+)=1��10-6 mol��L-1����Ksp[Al(OH)3]����ʽ�����c(OH-)=1��10-9��pH=5����5����ͼ�����������������������֪����a�ӵ�Դ���������������ĵ缫��ӦʽΪ��2H2O-4e-=O2��+4H+ ���۴Ӳ�Ʒ���ɽǶȷ�����֪��ԭ������[B(OH)4]-������Ĥ�����Ʒ�ң�Na+������Ĥ���������ҡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�