��Ŀ����
��ҵ���ô�����ͭ(������FeO)Ϊԭ����ȡ�Ȼ�ͭ����(CuCl2��2H2O)�������������£�

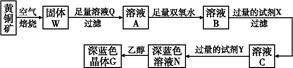
(1)д������ҺA�м���NaClO�����ӷ���ʽ___________________________________��
(2)����C�Ļ�ѧʽΪ________��
(3)ʵ������μ������E��ϴ�Ӹɾ���________��
(4)����������Ŀ����______________________________________��
(5)���������μ�����CuO��������һ��������ҺB�м��������CuO����������________________________________________________��

| ���� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ��ȫ����ʱ��pH | ��9.6 | ��6.4 | 3��4 |
(2)����C�Ļ�ѧʽΪ________��
(3)ʵ������μ������E��ϴ�Ӹɾ���________��
(4)����������Ŀ����______________________________________��
(5)���������μ�����CuO��������һ��������ҺB�м��������CuO����������________________________________________________��
(1)2Fe2����ClO����2H��=2Fe3����Cl����H2O
(2)Fe(OH)3
(3)ȡ���һ��ϴ��Һ�������Թ��У�����AgNO3��Һ���ް�ɫ�������ɣ�˵����ϴ�ɾ�
(4)����Cu2��ˮ�⣬��ֹ�����нᾧˮʧȥ(���һ�㼴��)
(5)һ���Լ��������CuO������Fe3����Cu2��ͬʱ���ɳ���
(2)Fe(OH)3
(3)ȡ���һ��ϴ��Һ�������Թ��У�����AgNO3��Һ���ް�ɫ�������ɣ�˵����ϴ�ɾ�
(4)����Cu2��ˮ�⣬��ֹ�����нᾧˮʧȥ(���һ�㼴��)
(5)һ���Լ��������CuO������Fe3����Cu2��ͬʱ���ɳ���
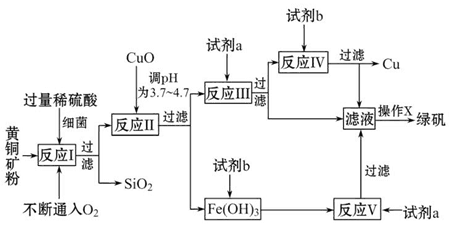
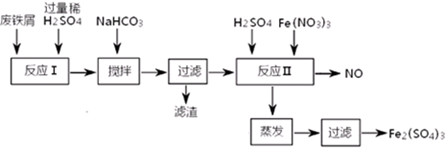
ͨ�������̵ķ�������ȷ��ҵ����ԭ��������������������塣�乤ҵ����ԭ��Ϊ���������ʵĴ�����ͭ�������ܽ⣬����������NaClO������Fe2������ΪFe3����Ȼ���ټ�����������ͭ������ҺpH��ʹFe3��ת��ΪFe(OH)3����ͨ�����˶���ȥ��Ȼ��������Һ�м�����������ͭ������ҺpH�����ȣ�ʹCu2��ת��ΪCuO�����CuO�������ᣬ���������õ��Ȼ�ͭ���塣

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д�
�����Ŀ
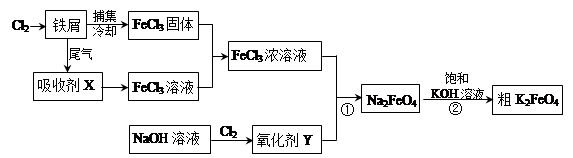
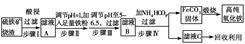
 4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ��� CrO42-��Fe(OH)3����OH-
CrO42-��Fe(OH)3����OH-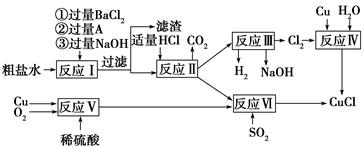
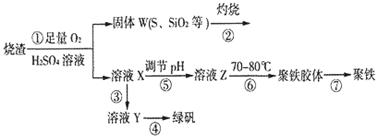
 [Cu(NH3)4]2++2OH-+4H2O,д���÷�Ӧ��ƽ�ⳣ������ʽ:������������������
[Cu(NH3)4]2++2OH-+4H2O,д���÷�Ӧ��ƽ�ⳣ������ʽ:������������������