��Ŀ����
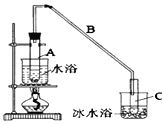
����Ŀ��ij��ѧС���������������������װ��(��ͼ)���Ի������Ʊ�����ϩ��
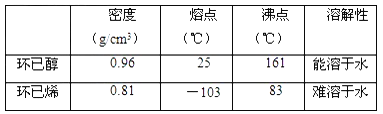
��֪��
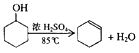
(1)�Ʊ���Ʒ��
��12.5 mL�����������Թ�A�У��ټ���l mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
�ٵ���B���˵�������е�������_____________________________________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����_____________________________________��
(2)�Ʊ���Ʒ��
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_____��(���ϻ���)����Һ����________(������)ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
�ڽ���Һ�õ��Ļ���ϩ�ٽ��������ռ���Ʒʱ�����Ƶ��¶�Ӧ��_______���ҡ�
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������___________��
a�������Ը��������Һ b���ý����� c���ⶨ�е�
���𰸡����� ��ֹ����ϩ�ӷ� �ϲ� c 83�� b c
��������
��1���ٵ���B���˵�������е�������������
������Ϣ��֪������ϩ�ķе�ϵͣ����Թ�C���ڱ�ˮԡ�е�Ŀ������ȴ����ϩ����ֹ����ϩ�Ļӷ���
��2���ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ������ˮ���ܶ�С��ˮ���ܶȣ�Ӧ���ϲ㣻��Һ���ÿ���Na2CO3��Һ��ȥ�������ʣ���ѡc��
�ڻ���ϩ�ķе���83�棬���ռ���Ʒʱ�����Ƶ��¶�Ӧ��83�����ң�
��3������ϩ��Ʒ�к��л������������������ʵȣ����ý����������𣻻����û�й̶��ķе㣬���������й̶��ķе㣬���ͨ���ⶨ�е���𣬴�ѡb c��

 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�


