��Ŀ����
����ѧ����ѡ��2����ѧ�뼼������15�֣�
���������漰ú�ڻ��������е�Ӧ�ã�
��1��ú��ת����������ú������������Һ��������
ú��Һ�������ַ�Ϊ ��
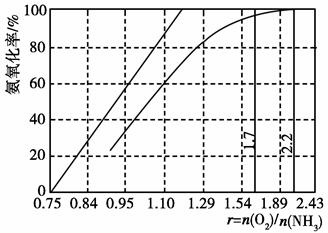
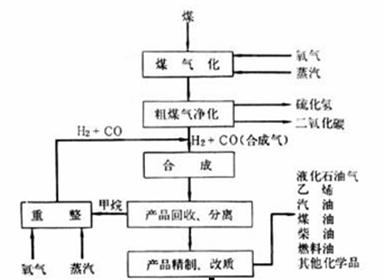
��2����úȼ��ǰ���ú������������ú��ij����������ԭ������ͼ��ʾ��
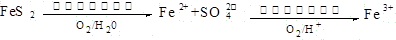
������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ ���ڶ�����Ӧ�����ӷ���ʽΪ ��
��3����ҵú����õ��IJ�Ʒ�н�̿�� �� �ȡ�
��4��ʪʽʯ��ʯ��ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������Һ�Ӵ���ͨ�����������ʯ�࣬�������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
��д��ʪ��ʯ��ʯ��ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��____________________________��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�__________________________
�����������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)���� ���ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����___________________________________��
��5��ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ������(CaSO4��xH2O)���ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ��ͼ��ʾ�����ݱ��������������Ϊ2.72g���ٸı䡣��ʯ��Ļ�ѧʽΪ_______________����ͼ����AB�ζ�Ӧ������Ļ�ѧʽΪ_________________��
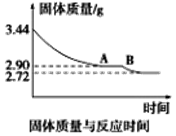
���������漰ú�ڻ��������е�Ӧ�ã�
��1��ú��ת����������ú������������Һ��������
ú��Һ�������ַ�Ϊ ��
��2����úȼ��ǰ���ú������������ú��ij����������ԭ������ͼ��ʾ��
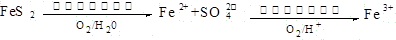
������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ ���ڶ�����Ӧ�����ӷ���ʽΪ ��
��3����ҵú����õ��IJ�Ʒ�н�̿�� �� �ȡ�
��4��ʪʽʯ��ʯ��ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������Һ�Ӵ���ͨ�����������ʯ�࣬�������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
��д��ʪ��ʯ��ʯ��ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��____________________________��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�__________________________
�����������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)���� ���ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����___________________________________��
��5��ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ������(CaSO4��xH2O)���ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ��ͼ��ʾ�����ݱ��������������Ϊ2.72g���ٸı䡣��ʯ��Ļ�ѧʽΪ_______________����ͼ����AB�ζ�Ӧ������Ļ�ѧʽΪ_________________��
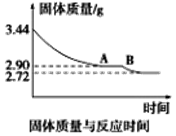
��15�֣���1��ֱ��Һ������ ��1�֣� ���Һ��������1�֣�
��2��2FeS2+7O2+2H2O=4H++2Fe2++4SO42-��2�֣�
4Fe2++O2+4H+=4Fe3++2H2O (2��)
��3����¯ú�����ְ�ˮ��ú���ͣ� ��2�֣�
��4����CaCO3+SO2=CaSO3+CO2 ��1�֣� 2CaSO3+O2=2CaSO4 ��1�֣�
����ʯ��ʯ��Һ�ijɱ��ϵͣ�1�֣� ����ˮϴ�ӣ�1�֣�
��5����CaSO4.2H2O(1��) ��2CaSO4. H2O��2�֣�
��2��2FeS2+7O2+2H2O=4H++2Fe2++4SO42-��2�֣�
4Fe2++O2+4H+=4Fe3++2H2O (2��)
��3����¯ú�����ְ�ˮ��ú���ͣ� ��2�֣�
��4����CaCO3+SO2=CaSO3+CO2 ��1�֣� 2CaSO3+O2=2CaSO4 ��1�֣�
����ʯ��ʯ��Һ�ijɱ��ϵͣ�1�֣� ����ˮϴ�ӣ�1�֣�
��5����CaSO4.2H2O(1��) ��2CaSO4. H2O��2�֣�
�����������1��ú��Һ�������ַ�Ϊֱ��Һ�������� ���Һ������
��2��FeS2�����������£���������ˮ��Ӧ������Fe2+��4SO42-���������ӷ���ʽΪ2FeS2+7O2+2H2O=4H++2Fe2++4SO42-�����������������������Fe3+�����ӷ���ʽΪ4Fe2++O2+4H+=4Fe3++2H2O��
��3����ҵú����õ��IJ�Ʒ�н�̿����¯ú�����ְ�ˮ��ú���͵ȣ�
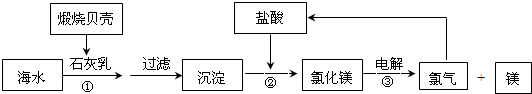
��4����ʪ��ʯ��ʯ��ʯ�෨�������У�̼������������Ӧ����������ƣ���������ٱ�������������ʯ�࣬��ѧ����ʽ��SO2+CaCO3=CaSO3+CO2��2CaSO3+O2+4H2O=2��CaSO4?2H2O����
��ʯ��ʯ��Һ������Ȼ��ֱ�Ӽӹ��õ����۸�ͣ���ʯ����Ҫ��ʯ��ʯ��ȡ��
����Ϊ�ǿ������Ȼ��ʯ�����ˮ�����Կ������Ȼ���ѡ����ˮϴ�ķ������ȽϾ���ʵ�ݣ�
��5���ٹ������������3.44g����Ϊ2.72gʱ����������0.72g��֮������������ٱ仯��˵��ԭ������CaSO4�����ʵ�����2.72g/136g/mol=0.02mol��ˮ�����ʵ�����0.72g/18g/mol=0.04mol�����Զ��ߵ����ʵ���֮����1:2����ʯ��Ļ�ѧʽΪCaSO4?2H2O��
��A���Ӧ����������2.90g��B���Ӧ������2.72g����ٵļ��㷽����ͬ��˵��A������CaSO4�����ʵ�����2.72g/136g/mol=0.02mol��ˮ�����ʵ����ǣ�2.90-2.72��g/18g/mol=0.01mol�����Զ��ߵ����ʵ���֮����2:1����ʯ��Ļ�ѧʽΪ2CaSO4?H2O��

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�����Ŀ
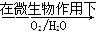 Fe2++SO42-
Fe2++SO42-