��Ŀ����
������˹����̽��ú̿����1496�ڶ�֣�Լռȫ���ܴ�����1/6��ú����Ҫ����Դ��Ҳ������������Ʒ����Ҫԭ�ϡ�������ѧ֪ʶ������������⡣
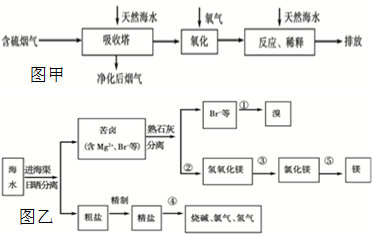
��1��ú��ת����������ú������������Һ��������ú��Һ�������ַ�Ϊ �� ��
��2����úȼ��ǰ���ú������������ú��ij����������ԭ��Ϊ
FeS2 Fe2++SO42-
Fe2++SO42-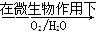 Fe3+
Fe3+
������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ ���ڶ�����Ӧ�����ӷ���ʽΪ ��
��3����ҵú����õ��IJ�Ʒ�н�̿�� ��
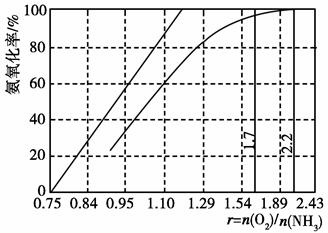
��4����ҵ����Ҫ���ð��������������ᣬ��ͼ�ǰ��������백������������������ȵĹ�ϵ������ֱ�߱�ʾ��Ӧ������ֵ�����߱�ʾ����ʵ����������������ʴﵽ100%��������r[n(O2)/n(NH3)]�� ��ʵ������Ҫ��rֵά����1.7��2.2֮�䣬ԭ���� ��
��1��ú��ת����������ú������������Һ��������ú��Һ�������ַ�Ϊ �� ��
��2����úȼ��ǰ���ú������������ú��ij����������ԭ��Ϊ
FeS2
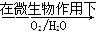 Fe2++SO42-
Fe2++SO42-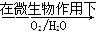 Fe3+
Fe3+������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ ���ڶ�����Ӧ�����ӷ���ʽΪ ��
��3����ҵú����õ��IJ�Ʒ�н�̿�� ��
��4����ҵ����Ҫ���ð��������������ᣬ��ͼ�ǰ��������백������������������ȵĹ�ϵ������ֱ�߱�ʾ��Ӧ������ֵ�����߱�ʾ����ʵ����������������ʴﵽ100%��������r[n(O2)/n(NH3)]�� ��ʵ������Ҫ��rֵά����1.7��2.2֮�䣬ԭ���� ��

��1��ֱ��Һ������ ���Һ��������
��2��2FeS2��7O2��2H2O 4H����2Fe2����4
4H����2Fe2����4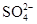 ��4Fe2����O2��4H��
��4Fe2����O2��4H�� 4Fe3����2H2O��
4Fe3����2H2O��
��3����¯ú�����ְ�ˮ��ú���ͣ���4��1.25��O2̫�ٲ�����NH3��ת����rֵΪ2.2ʱNH3�������ѽ�100%��
��2��2FeS2��7O2��2H2O
 4H����2Fe2����4
4H����2Fe2����4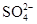 ��4Fe2����O2��4H��
��4Fe2����O2��4H�� 4Fe3����2H2O��
4Fe3����2H2O����3����¯ú�����ְ�ˮ��ú���ͣ���4��1.25��O2̫�ٲ�����NH3��ת����rֵΪ2.2ʱNH3�������ѽ�100%��
�����������2��ԭ��FeS2
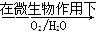 Fe2++SO42-
Fe2++SO42-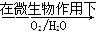 Fe3+��ѭ�����غ���ɣ����ò������д��������һ�� FeS2+ O2+ H2O
Fe3+��ѭ�����غ���ɣ����ò������д��������һ�� FeS2+ O2+ H2O Fe2++ SO42-+ �����ݵ���غ��ԭ���غ㣬��HдΪH+��
Fe2++ SO42-+ �����ݵ���غ��ԭ���غ㣬��HдΪH+����4����ͼ�п������������ʴﵽ100%��������r[n(O2)/n(NH3)]��1.25��ʵ������Ҫ��rֵά����1.7��2.2֮�䣬ԭ����O2̫�ٲ�����NH3��ת����rֵΪ2.2ʱNH3�������ѽ�100%��

��ϰ��ϵ�д�
�����Ŀ