��Ŀ����
�±�ΪԪ�����ڱ���һ���֡���ش��������⣺
����Ԫ�������ڱ��е����λ�ÿ�֪�١���ֱ���H��C��N��O��Mg��Al��Cl��Ca��Mn��Fe��
��1������Ԫ���У�����s������____________(��Ԫ�ط���)��
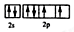
��2��д��Ԫ�آܵĻ�̬ԭ�ӵļ۵����Ų�ͼ____________________��
��3��Ԫ�ص�һ������Ϊ��________�� (����ڡ���С�ڡ�)��
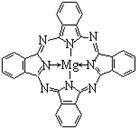
��4��Ԫ�آ���̬�⻯���VSEPRģ��Ϊ________���÷���Ϊ________����(����ԡ��Ǽ��ԡ�)��������ͭ��Һ����μ�����ˮ��Һ���ɹ۲쵽������Ϊ_____________________________��
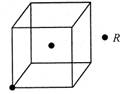
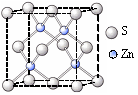
��5�����ʵľ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��
����֪��ԭ�Ӱ뾶Ϊdcm��NA���������ӵ�������Ԫ�آ����ԭ������ΪM����ش𣺾����Т�ԭ�ӵ���λ��Ϊ ,�þ�����ܶ�Ϊ ������ĸ��ʾ��
��6��ʵ��֤�����ݺ͢�������KCl��TiN��4�־���Ľṹ��NaCl����ṹ���ƣ�����ͼ��ʾ������֪3�����Ӿ���ľ������������±���
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |
���4�����Ӿ��壨������NaCl���۵�Ӹߵ��͵�˳���ǣ� ���û�ѧʽ��д����
���Т�������ᄃ����һ����������Χ�������ڽ��ҵȾ������������ ����
��1��H Mg Ca��2��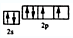 ��
�� ��3������
��3������
��4�������� ���� �Ȳ�����ɫ���������ܽ������ɫ��Һ
��5��12 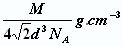 ��6��TiN��MgO��CaO��KCl 6
��6��TiN��MgO��CaO��KCl 6
�����������������Ԫ�������ڱ��е����λ�ÿ�֪�١���ֱ���H��C��N��O��Mg��Al��Cl��Ca��Mn��Fe��
��1���������������ڰ��չ���ԭ�����������ӵĹ�����ƣ����Ը���Ԫ�صĺ�������Ų���֪������Ԫ���У�����s������H��Mg��Ca��
��2����Ԫ�ص�������������6�������Ի�̬ԭ�ӵļ۵����Ų�ͼ�� ��
�� ��
��
��3������MnԪ�ص�3d�ܼ���5�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܴ�������Ԫ�صģ�����MnԪ�صĵ�һ�����ܴ�����Ԫ�صĵ�һ�����ܡ�
��4�����ݼ۲���ӶԻ������ۿ�֪�����������е�Ԫ�غ��еŶԵ��Ӷ�������5��3��1����2��1����������VSEPRģ��Ϊ�����壻��ʵ�ʵĿռ乹���������Σ����Ը÷���Ϊ���Է��ӡ�����ͭ�����ܺͰ����γ���λ��������������ͭ��Һ����μ�����ˮ��Һ���ɹ۲쵽������Ϊ�Ȳ�����ɫ���������ܽ������ɫ��Һ��
��5�����ݽ������ľ�����֪���þ�������λ���ǣ�3��8����2��12��������������ԭ�ӵĸ����� �����ݱ��Ľṹ��֪���þ����ı߳���
�����ݱ��Ľṹ��֪���þ����ı߳���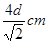 ��������
��������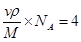 ,��˾������ܶ�
,��˾������ܶ�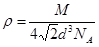 g/cm3��
g/cm3��
��6�����Ӿ�������Ӱ뾶ԽС���������Խ�࣬������Խ��������۷е�Խ�ߣ�����TiN��MgO��MgO��CaO���ɱ������ݿ�֪CaO��KCl������۵�Ӹߵ��͵�˳����TiN��MgO��CaO��KCl���Ȼ��Ƶ���λ����6���������¡����Һ�ǰ���1��������Ca�������ᄃ����һ����������Χ�������ڽ��ҵȾ������������6����
���㣺����Ԫ�����ڱ��Ľṹ���۵����Ų�ͼ����λ������һ�����ܡ����ӵĿռ乹���Լ��������͵��йؼ��㡢�жϺ��۵�Ƚϵ�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ��������������������������Ҫ��Ԫ�ء�λ�������ԡ����߹�ϵ���ۺϿ��飬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ��������������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������������ѵ��Ǿ����ܶȵļ��㡣

 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д��±���Ԫ�����ڱ���һ���֣�����Ҫ��ش��������⡣
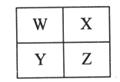
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 2 | | | | E | H | F | I | |
| 3 | A | C | D | | | | G | R |
| 4 | B | | | | | | | |
��1��ʮ��Ԫ���л�ѧ��������õ�Ԫ����________����Ԫ�ط��ţ���
��2��A��C��D����Ԫ�ص��������Ӧ��ˮ������м�����ǿ����________���ѧʽ����
��3��IԪ�ظ�AԪ���γɻ�����ĵ���ʽ��________���������ոû�����ʱ�������________ɫ��
��4��G�ĵ��ʺ�B������������Ӧˮ���ﷴӦ�����ӷ���ʽ��__________________��
Ԫ��A��F���γ����ֻ����д�����н��ȶ��Ļ�������CO2��Ӧ���������Ļ�ѧ����ʽ ________________________��
��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA<C<B<D<E����֪Aԭ�ӵ�p���Ϊ����������γɵļ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵġ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B�����ӱ�Dԭ���γɵ�������һ�����Ӳ㡣C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29��
��ش��������⣺
(1) Ԫ��A���⻯����Aԭ�ӵ��ӻ�������________��B��C��D�ĵ縺����С�����˳��Ϊ______(������Ӧ��Ԫ�ط��ű�ʾ)��C����̬�⻯��������ˮ��ԭ����____________________��
(2)Eԭ�ӵĻ�̬�����Ų�ʽΪ________��Ԫ��E�ĵ��ʾ����ڲ�ͬ�¶��¿������ֶѻ���ʽ�������ֱ���ͼa��b��ʾ���������������ѻ��ľ��������������ѻ��ľ�����ʵ�ʺ��е�Eԭ�ӵĸ���֮��Ϊ____________��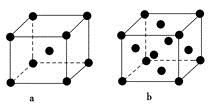
(3)ʵ��֤����KCl��MgO��CaO��TiN��4�־���Ľṹ��NaCl����ṹ����(��ͼ��ʾ)������3�����Ӿ���ľ������������±���
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |
(4)���������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ���________��
(5)����ЧӦ����ѧ����Ʒ�Ӧ��CO2��4H2����CH4��2H2O�Լ�С������CO2������1 mol CH4���ɣ�����________mol �Ҽ���________mol �м����ѡ�


 4AC2(g)��B2(g)�����������£��������г���AC��BC2��1 mol��ƽ��ʱ������AC2��B2��a mol����AC��ת������________(�ú�a�Ĵ���ʽ��ʾ)��
4AC2(g)��B2(g)�����������£��������г���AC��BC2��1 mol��ƽ��ʱ������AC2��B2��a mol����AC��ת������________(�ú�a�Ĵ���ʽ��ʾ)��