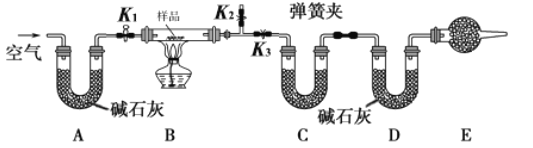
��Ŀ����
����Ŀ����֪X��һ�־��й���ζ�ĺϳ����ϣ���ͼΪ�ϳ�X��ij�����̣�

��ʾ����![]() �������ձ�����Ϊ��COOH��
�������ձ�����Ϊ��COOH��
��D�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��
�����������Ϣ���ش��������⣺
(1)C�����й����ŵ�������__________��E�Ľṹ��ʽ��________��
(2)D+E��X�Ļ�ѧ��Ӧ����Ϊ________��Ӧ��
(3)����A��B��C��D��E��X���������У���Ϊͬϵ�����____________________��
(4)C��һ��ͬ���칹��F���Է���ˮ�ⷴӦ����F�Ľṹ��ʽΪ________�� ________��
(5)��ӦC��E��X�Ļ�ѧ����ʽΪ______________��
���𰸡� �Ȼ� CH3CH2OH ������Ӧ/ȡ����Ӧ A��E HCOOCH2CH3 CH3COOCH3 CH3CH2COOH + CH3CH2OH ![]() CH3CH2COOCH2CH3 + H2O
CH3CH2COOCH2CH3 + H2O
��������D�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��DΪ��ϩ����ϩ��ˮ�����ӳɱ�Ϊ�Ҵ���E��������X�Ǻ���5��̼�ı���һԪ���������л���CΪ����3��C�ı���һԪ���ᣬ��������ԣ��л���AΪ1-�������л���BΪ��ȩ��
(1)����������CΪ���ᣬ�����ŵ������Ȼ���EΪ�Ҵ����ṹ��ʽCH3CH2OH����ȷ�𰸣��Ȼ���CH3CH2OH��
(2)�Ҵ��ͱ��ᷢ��������Ӧ��������ˮ����ȷ�𰸣�������Ӧ/ȡ����Ӧ��
(3)1-��������ȩ�����ᡢ��ϩ�������������Ҵ����������У���Ϊͬϵ�����1-�������Ҵ�����ȷ�𰸣�A��E��
(4) FΪ�����ͬ���칹�壬�ܹ�����ˮ�ⷴӦ���ṹ�к���������F�Ľṹ��ʽΪ�ֱ�Ϊ��HCOOCH2CH3 ��CH3COOCH3����ȷ�𰸣�HCOOCH2CH3 ��CH3COOCH3��
(5) ������Ҵ���Ũ������������·���������Ӧ����ѧ����ʽΪCH3CH2COOH + CH3CH2OH ![]() CH3CH2COOCH2CH3 + H2O����ȷ�𰸣�CH3CH2COOH + CH3CH2OH
CH3CH2COOCH2CH3 + H2O����ȷ�𰸣�CH3CH2COOH + CH3CH2OH ![]() CH3CH2COOCH2CH3 + H2O��
CH3CH2COOCH2CH3 + H2O��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�����Ŀ��(1)2017���п�Ժij�о��Ŷ�ͨ�����һ������Na-Fe3O4/HZSM-5��ܸ��ϴ������ɹ�ʵ����CO2ֱ�Ӽ�����ȡ����ֵ���ͣ����о��ɹ�������Ϊ��CO2��ת�������ͻ���Խ�չ����
��֪��H2(g)+1/2O2(g)=H2O(l) ��H1 = ��aKJ/mol
C8H18(1)+25/2O2(g)=8CO2(g)+9H2O(1) ��H2= ��bKJ/mol
��д��25����101kPa�����£�CO2��H2��Ӧ��������(��C8H18��ʾ)���Ȼ�ѧ����ʽ_________________________________��
(2)����CO2��H2Ϊԭ�ϣ��ں��ʵĴ���(��Cu/ZnO����)�����£�Ҳ�ɺϳ�CH3OH���漰�ķ�Ӧ�У�
�ף�CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H= �� 53.7kJ��mol-1 ƽ�ⳣ��K1
CH3OH(g)+H2O(g) ��H= �� 53.7kJ��mol-1 ƽ�ⳣ��K1
�ң�CO2(g)+H2(g) ![]() CO(g)+H2O(g) ��H= + 41.2kJ��mol-1 ƽ�ⳣ��K2
CO(g)+H2O(g) ��H= + 41.2kJ��mol-1 ƽ�ⳣ��K2
��CO(g)+2H2(g) ![]() CH3OH(g)��ƽ�ⳣ��K=______(�ú�K1��K2�ı���ʽ��ʾ)���÷�Ӧ��H_____0(��������������С����)��
CH3OH(g)��ƽ�ⳣ��K=______(�ú�K1��K2�ı���ʽ��ʾ)���÷�Ӧ��H_____0(��������������С����)��
�����CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��___________(��д����)��
�۴����ͷ�Ӧ��ϵ�Ĺ�ϵ��������Կ�Ĺ�ϵһ�������и߶ȵ�ѡ���ԡ���������ʵ�飬����CO2��H2��ʼͶ�ϱȾ�Ϊ1��2.2��������ͬ��Ӧʱ��(t1min)��
�¶�(K) | ���� | CO2ת����(%) | �״�ѡ����(%) | �ۺ�ѡ�� |
543 | Cu/ZnO���װ����� | 12.3 | 42.3 | A |
543 | Cu/ZnO����Ƭ���� | 11.9 | 72.7 | B |
553 | Cu/ZnO���װ����� | 15.3 | 39.1 | C |
553 | Cu/ZnO����Ƭ���� | 12.0 | 70.6 | D |
�ɱ����е����ݿ�֪����ͬ�¶��²�ͬ�Ĵ�����CO2��ת��ΪCH3OH��ѡ����������Ӱ�죬�����ϱ��������ݽ�Ϸ�Ӧԭ������������ѡ��Ϊ___________(����ĸ����)��
(3)��CO��H2Ϊԭ�Ϻϳ��״��ķ�ӦΪ��CO(g)+2H2(g)![]() CH3OH(g)���������Ϊ2L�����������ܱ����������������У��ֱ���1molCO��2molH2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䡣��ͼΪ���������еķ�Ӧ�����е�5minʱH2���������ʾ��ͼ��������һ��������Ӧһ���ﵽƽ��״̬��
CH3OH(g)���������Ϊ2L�����������ܱ����������������У��ֱ���1molCO��2molH2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䡣��ͼΪ���������еķ�Ӧ�����е�5minʱH2���������ʾ��ͼ��������һ��������Ӧһ���ﵽƽ��״̬��
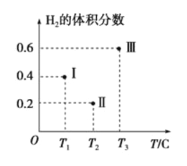
��0��5minʱ��������������CH3OH��ʾ�Ļ�ѧ��Ӧ����Ϊ_________________��
������������һ���ﵽƽ��״̬��������________(��д��������)��