��Ŀ����
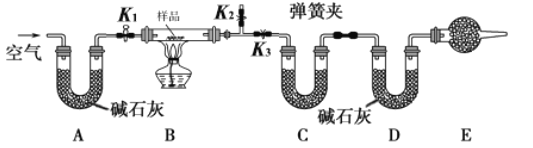
����Ŀ����һ��NaCl��Na2CO3��10H2O��NaHCO3�Ļ���ijͬѧ�������ʵ�飬ͨ��������Ӧǰ��C��Dװ�������ı仯���ⶨ�û�����и���ֵ�����������
��1���뽫ʵ�鲽�貹������
�ٰ�ͼ���г�����δ��������װ��ʵ��װ�ú����Ƚ��еIJ����� _____________________��
�ڳ�ȡ��Ʒ�����������Ӳ�ʲ������У�������C��Dװ�õ�������
�۴���K1��K2���ر�K3������������������ӣ���Ŀ����____________________��
�ܹرջ���K1��K2����K3����ȼ�ƾ��Ƽ��������ٲ������塣
�ݴ���K1������������������ӣ�Ȼ��ж��װ�ã��ٴγ���C��Dװ�õ�������
��2�����ڸ�ʵ�鷽������ش��������⣺
�������ȷ�Ӧ�����������NaCl�ⶨ�����Ӱ����___________����ƫ��������ƫ����������Ӱ������
��E���������ʢ�ŵ�ҩƷ��_______________����������_____________________________�����ʵ����û�и�װ�ã���ᵼ�²������NaHCO3����������______________����ƫ��������ƫ����������Ӱ������
������Ʒ����Ϊwg����Ӧ��C��Dװ�����ӵ������ֱ�Ϊm1g��m2g���ɴ˿�֪�������Na2CO3��10H2O����������Ϊ______���ú�w��m1��m2�Ĵ���ʽ��ʾ����
���𰸡����װ�������� ����װ���к��е�ˮ�����Ͷ�����̼��������� ƫ�� ��ʯ�� �����������еĶ�����̼��ˮ���� ƫ�� ![]() ����m1-
����m1-![]() m2����100%
m2����100%
��������
��NaCl��Na2CO310H2O��NaHCO3�Ļ�������ʱ��̼�����Ʒֽ�����̼���ơ�������̼��ˮ��̼���ƾ���ʧȥ�ᾧˮ����̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3![]() Na2CO3+H2O��+CO2����Na2CO310H2O
Na2CO3+H2O��+CO2����Na2CO310H2O![]() Na2CO3+10H2O����������H2O(g)��CO2��Ӧ��C��D�зֱ����գ��ɸ����������֪Ӧ������ˮ�������ն�����̼����C�еĸ������ˮ����������CO2����D������(NaHCO3�ֽ������CO2������)�����NaHCO3��������C������(Na2CO310H2O�ֽ������H2O���Ѿ�֪����NaHCO3�ֽ������H2O������)�����Na2CO310H2O���������Ӷ����NaCl���������ݴ˷������
Na2CO3+10H2O����������H2O(g)��CO2��Ӧ��C��D�зֱ����գ��ɸ����������֪Ӧ������ˮ�������ն�����̼����C�еĸ������ˮ����������CO2����D������(NaHCO3�ֽ������CO2������)�����NaHCO3��������C������(Na2CO310H2O�ֽ������H2O���Ѿ�֪����NaHCO3�ֽ������H2O������)�����Na2CO310H2O���������Ӷ����NaCl���������ݴ˷������
(1)����ʵ��ԭ����֪��ʵ����Ҫͨ������Dװ���ڼ�ʯ�ҵ����أ��������ɵĶ�����̼��������ͨ������Cװ���ڵ����أ��������ɵ�ˮ����������Ӧ���ȼ���װ�õ������ԣ��ʴ�Ϊ�����װ�������ԣ�
��װ�����п����������к���ˮ�����Ͷ�����̼��Ӱ��ˮ�����Ͷ�����̼�����IJⶨ������K1��K2���رջ���K3��ʵ��ǰҪͨ�����������װ���к���ˮ�����Ͷ�����̼���������ʴ�Ϊ����ȥװ���е�ˮ�����Ͷ�����̼��
(2)�������ȷ�Ӧ���������ʹ��ˮ�����Ͷ�����̼�������ⶨ������С��̼���������ݶ�����̼���㣬��Na2CO310H2O�IJⶨ�Ǹ�������ˮ������������ģ������NaHCO3��Na2CO310H2O�ĺ�����ƫС����NaCl�IJⶨ�����ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��E��������Ƿ�ֹ�������е�ˮ�����Ͷ�����̼Ӱ��ʵ��������˸������ʢ�ż�ʯ�ң���Ϊ��ʯ�������տ����е�ˮ�����Ͷ�����̼����û��Eװ�ã���ⶨ��̼�����Ƶ�����ƫ�ߣ��ʴ�Ϊ����ʯ�ң������������еĶ�����̼��ˮ������ƫ�ߣ�
�ۺ�NaCl��Na2CO310H2O��NaHCO3�Ļ�������ʱ��̼�����Ʒֽ�����̼���ơ�������̼��ˮ��̼���ƾ���ʧȥ�ᾧˮ����̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3![]() Na2CO3+H2O��+CO2����Na2CO310H2O
Na2CO3+H2O��+CO2����Na2CO310H2O![]() Na2CO3+10H2O����Dװ�������ӵ�����Ϊ������̼����������̼�����Ʒֽ����ɵ�ˮ����������Ϊx��
Na2CO3+10H2O����Dװ�������ӵ�����Ϊ������̼����������̼�����Ʒֽ����ɵ�ˮ����������Ϊx��
2NaHCO3![]() Na2CO3+H2O + CO2��
Na2CO3+H2O + CO2��
18g 44g
x m2g
![]() =
=![]() �����x=
�����x=![]() m2g
m2g
װ��C���յ���ˮ����������̼�����Ʒֽ����ɵĺ�Na2CO310H2O�ֽ����ɵ�ˮ����Na2CO310H2O�ֽ����ɵ�ˮ����������=m1g -![]() m2g =(m1-
m2g =(m1-![]() m2)g����Na2CO310H2O������Ϊy��
m2)g����Na2CO310H2O������Ϊy��
Na![]() Na2CO3+10H2O
Na2CO3+10H2O
286g 180g
y (m1-![]() m2)g
m2)g
![]() =
=![]() ����ã�y=
����ã�y=![]() ��(m1-
��(m1-![]() m2)g������Na2CO3��10H2O����������=
m2)g������Na2CO3��10H2O����������=![]() ��100%=
��100%=![]() ��(m1-
��(m1-![]() m2)��100%���ʴ�Ϊ��
m2)��100%���ʴ�Ϊ��![]() ��(m1-
��(m1-![]() m2)��100%��
m2)��100%��