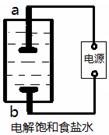
��Ŀ����
ʵ������Na2SO3������70�������ᷴӦ��ȡSO2����ʱ������FeC13��Һ���ն����SO2���塣
��1��д��SO2��FeC13��Һ��Ӧ�����ӷ���ʽ�� ��
��2��FeC13��Һ����SO2����һ��ʱ�������Һ��һ�����ڵ������У�H+��Fe2+��C1����SO42-��ijͬѧ��Ϊ�����ܴ����������ӣ�������������ʵ��̽����
������������裺
����1��������HSO3-��SO32-
����2��������Fe3+
����3��HSO3-��SO32-��Fe3+��������
�����ʵ�鷽��
| ʵ�鲽�� | ����ͽ��� |
| ����1��ȡ��������Һ���Թܣ��μ�ϡ�����ữ��Ȼ���ٵ��뼸�� ��Һ�� | ���� ���ۣ�����1�������� |
| ����2�� | ���� ���ۣ�����2������ |
��3���ⶨ������SO2�����ķ����ǣ���500L��SO2����Ŀ���ͨ��20mL��0.00015molKMnO4����Һ�У���ַ�Ӧ������0.02000mol/L��KI��Һ�ζ�������KMnO4������KI ��Һ25.00mL��������е�SO2����Ϊ mg/L��
��5SO2+2MnO4-+2H2O��2Mn2++5SO42-+4H+��10I��+2MnO4-+16H+��2Mn2++5I2+8H2O��
��1��SO2+2Fe3++2H2O=SO42��+2Fe2++4H+
��2������Ʒ����Һ Ʒ�첻��ɫ ȡ��������Һ���Թܣ��μ�����KSCN��Һ ��Һ���
��3��0.016mg/L
�����������������Fe3+�������Ժ�+4��S�Ļ�ԭ��֮�䷢����Ӧ�IJ������̽��������SO32-��HSO3-��Fe2+��Fe3+�ļ��飬SO32-��HSO3-������ת��ΪSO2��ͨ��Ʒ����Һ�����飬Fe3+���飬������KSCN�����顣���ݷ�Ӧ10I��+2MnO4-+16H+��2Mn2++5I2+8H2O���������������ص����ʵ���Ϊ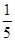 ��0.02000mol/L��0.025L=0.0001mol����֪������һ����Ӧ�����ĵĸ������Ϊ0.00015mol-0.0001mol=5��10-5mol��n(SO2)= 1.25��10-4mol������Ϊ1.25��10-4mol��64g/mol/500L=0.16��10-4g/L=0.016mg/L��
��0.02000mol/L��0.025L=0.0001mol����֪������һ����Ӧ�����ĵĸ������Ϊ0.00015mol-0.0001mol=5��10-5mol��n(SO2)= 1.25��10-4mol������Ϊ1.25��10-4mol��64g/mol/500L=0.16��10-4g/L=0.016mg/L��
���㣺������̽��ʵ��Ϊ������������������ԭ��Ӧ�����ʵļ��顢��ѧ��������֪ʶ��

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�Cl2�Ƿ�֯��ҵ�г��õ�Ư����Na2S2O3����ΪƯ�ײ�ƥ��ġ����ȼ����� S2O32-��Cl2��Ӧ�IJ���֮һΪSO42һ������˵���У�������� ( )
| A���÷�Ӧ�е���������C12 |
| B��SO2����ˮ��Ư��ԭ����ͬ�����Կ���S02����֯��ҵ��Ư�� |
| C��������Ӧ�У�ÿ����1 mol SO42һ������ȥ2 mol C12 |
| D�����ݸ÷�Ӧ���жϻ�ԭ�ԣ�S2O32->C1�� |
���������������ļӿ죬�����С��װʳƷ�ѱ��㷺���ܡ�Ϊ���ӳ�ʳƷ�ı����ڣ���ֹʳƷ�ܳ�����֬ʳƷ�������ʣ��ڰ�װ����Ӧ����Ļ�ѧ������
| A����ˮ����ͭ������ | B���轺���������� |
| C��ʳ�Ρ��������� | D����ʯ�ҡ�ʳ�� |
��֪��2KMnO4��16HCl===2KCl��2MnCl2��5Cl2����8H2O��Cl2��2FeCl2===2FeCl3��
2KI��2FeCl3===2KCl��I2��2FeCl2���������жϴ������
A�������ԣ�MnO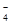 >Cl2>Fe3�� >I2 >Cl2>Fe3�� >I2 |
| B��FeCl3�������������л�ԭ�� |
| C����FeI2��Һ��ͨ��������Cl2��������Ӧ�ķ���ʽΪ6FeI2+3Cl2=2FeCl3+4FeI3 |
| D��FeCl3��ʹʪ��ĵ��۵⻯����ֽ���� |