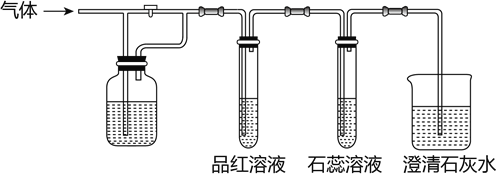
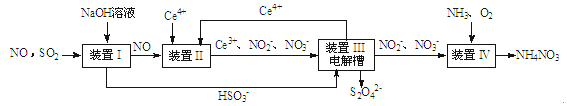
��Ŀ����
����Ŀ��A (��Ȳ���ǻ����л�����ԭ�ϡ���A�Ʊ��߷��ӿɽ������ϣ�PVB����IR�ĺϳ�·�ߣ����ַ�Ӧ������ȥ����ͼ��ʾ��
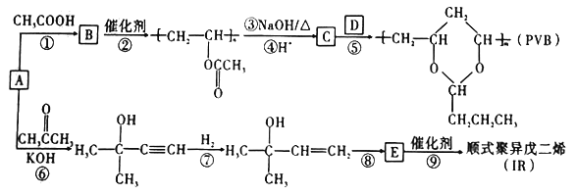
�ش��������⣺
��1��ʵ�����Ʊ�A�Ļ�ѧ��Ӧ����ʽΪ ____________________________________��
��2�����̢ٵķ�Ӧ����Ϊ____________��B�к��������ŵ�������____________________��
��3����Ӧ�۵Ļ�ѧ����ʽΪ_____________________________________����Ӧ��Ļ�ѧ����ʽΪ_____________________________________��
��4�����ڹ��̢ڣ�����˵����ȷ����_____������ţ���
a.��Ӧ���������۷�Ӧ b.�ø߷��Ӳ��Ͽ�������������֯��
c.�ø߷������ھ��к��䵥��һ���Ľṹ d.���ɵĸ߷��ӻ�������������ԣ���Ϊ�����
e.ͨ�������Dz�øø߷��ӵ�ƽ����Է�������Ϊ30444������֪��nԼΪ354
��5��D��IR�Ľṹ��ʽ�ֱ���_____________________��__________________��
��6������̢ߵIJ��ﺬ��ͬ�����ŵ�ͬ���칹����____�֡�����֪̼̼˫�����ǻ�ֱ���������ȶ���
��7����֪��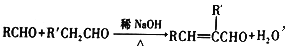 ����R��R����ʾ�������⣩����AΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�D��д���ϳ�·��_____________________���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
����R��R����ʾ�������⣩����AΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�D��д���ϳ�·��_____________________���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
���𰸡� CaC2+2H2O��Ca(OH)2+C2H2 �ӳɷ�Ӧ ���� 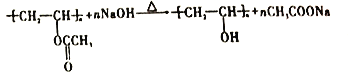
 de CH3CH2CH2CHO
de CH3CH2CH2CHO 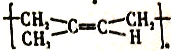 16
16 ![]()
���������ɷ���ʽ��֪AΪHC��CH�������ᷢ���ӳɷ�Ӧ����BΪCH2=CHOOCCH3�������Ӿ۷�Ӧ���� ��ˮ������CΪ
��ˮ������CΪ![]() ����PVB��֪DΪCH3CH2CH2CHO��HC��CH���ͪ��KOH�����·�Ӧ����
����PVB��֪DΪCH3CH2CH2CHO��HC��CH���ͪ��KOH�����·�Ӧ���� �������������ӳɷ�Ӧ����
�������������ӳɷ�Ӧ���� ��������ȥ��Ӧ���������ϩ�������ϩ�ڴ������·����Ӿ۷�Ӧ����˳ʽ�������ϩ����
��������ȥ��Ӧ���������ϩ�������ϩ�ڴ������·����Ӿ۷�Ӧ����˳ʽ�������ϩ����
��1��ʵ�����Ʊ���Ȳ�Ļ�ѧ��Ӧ����ʽΪ CaC2+2H2O��Ca(OH)2+C2H2��
�����Ϸ�����֪AΪ��Ȳ��BΪCH2=CHOOCCH3�����еĹ�����Ϊ̼̼˫�����������ʴ�Ϊ����Ȳ��̼̼˫������������2���������Ϸ�����֪���̢ٵķ�Ӧ����Ϊ�ӳɷ�Ӧ��BΪCH2=CHOOCCH3�����к��������ŵ���������������3���������Ϸ�����֪��Ӧ�۵Ļ�ѧ����ʽΪ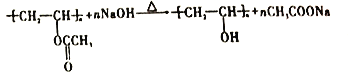 ����Ӧ��Ļ�ѧ����ʽΪ
����Ӧ��Ļ�ѧ����ʽΪ ����4��a.��Ӧ�����ڼӾ۷�Ӧ��a����b.�����к���������û����ˮ���ţ�û����ˮ�ԣ��ø߷��Ӳ��ϲ���������������֯����b����c.�ø߷����ǼӾ۲�������к���̼̼˫���������ڲ����к��䵥��һ���Ľṹ��c����d.�Ӿ۵õ����߷��ӣ�������ɵĸ߷��ӻ�������������ԣ���Ϊ�������d��ȷ��e.ͨ�������Dz�øø߷��ӵ�ƽ����Է�������Ϊ30444������B����Է���������86������n��30444��86��354��e��ȷ����ѡde����5���������Ϸ�����֪D��IR�Ľṹ��ʽ�ֱ���CH3CH2CH2CHO��
����4��a.��Ӧ�����ڼӾ۷�Ӧ��a����b.�����к���������û����ˮ���ţ�û����ˮ�ԣ��ø߷��Ӳ��ϲ���������������֯����b����c.�ø߷����ǼӾ۲�������к���̼̼˫���������ڲ����к��䵥��һ���Ľṹ��c����d.�Ӿ۵õ����߷��ӣ�������ɵĸ߷��ӻ�������������ԣ���Ϊ�������d��ȷ��e.ͨ�������Dz�øø߷��ӵ�ƽ����Է�������Ϊ30444������B����Է���������86������n��30444��86��354��e��ȷ����ѡde����5���������Ϸ�����֪D��IR�Ľṹ��ʽ�ֱ���CH3CH2CH2CHO�� ����6������̢ߵIJ��ﺬ��ͬ�����ŵ�ͬ���칹��Ӧ�ú���̼̼˫�����ǻ����������C=C-C-C-C���ǻ���λ����3�֣�����C-C=C-C-C���ǻ���λ����3�֣��Ҿ�����˳���칹�壬������C=C-C(CH3)2���ǻ���λ����2�֣�������C=C(CH3)-C-C���ǻ���λ����3�֣�������C-C=C(CH3)2���ǻ���λ����2�֣�����һ�ִ���˳���칹�壬�Ͻ���17�֣����Ի���16�֡���7��������֪��Ϣ��������Ʒ���֪��AΪ��ʼԭ�ϣ��ϳ�D�ĺϳ�·��Ϊ
����6������̢ߵIJ��ﺬ��ͬ�����ŵ�ͬ���칹��Ӧ�ú���̼̼˫�����ǻ����������C=C-C-C-C���ǻ���λ����3�֣�����C-C=C-C-C���ǻ���λ����3�֣��Ҿ�����˳���칹�壬������C=C-C(CH3)2���ǻ���λ����2�֣�������C=C(CH3)-C-C���ǻ���λ����3�֣�������C-C=C(CH3)2���ǻ���λ����2�֣�����һ�ִ���˳���칹�壬�Ͻ���17�֣����Ի���16�֡���7��������֪��Ϣ��������Ʒ���֪��AΪ��ʼԭ�ϣ��ϳ�D�ĺϳ�·��Ϊ![]() ��
��

 ������������ϵ�д�
������������ϵ�д�