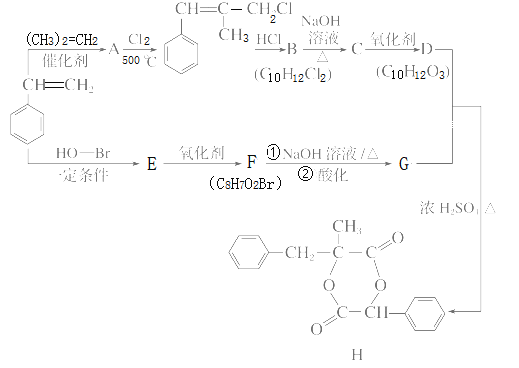
��Ŀ����
����Ŀ��X��Y��Z��W��Ϊ������Ԫ����ԭ��������������Ԫ��Y��Wͬ�塣��ZWX��Ũ���ᷴӦ���л���ɫ���������������ͬ���ռ���Һ���ã��ɵõ���ZWX����Һ������������Ϣ�����ж��������⣺
��1��д��������Ԫ�ص�Ԫ�ط��ţ�X_____��Y_____��Z____��W____������������Ԫ�ص�ԭ�Ӱ��հ뾶�ɴ�С��˳������______���þ����Ԫ�ط�������
��2��Y���⻯���ȶ���_________W���⻯���ȶ��ԣ�����ڡ���С�ڡ�����Y���⻯��ˮ��Һ����_________W���⻯��ˮ��Һ�����ԣ�����ڡ���С�ڡ�����
��3��д��Z2X2�ĵ���ʽ_____��������������ѧ����������_________��0.1 mol��������ˮ��Ӧת�Ƶ�����Ϊ______��
���𰸡�O F Na Cl Na > Cl > O > F ���� С�� ![]() ���Ӽ����Ǽ��Լ� 0.1NA���� 6.02��1022����
���Ӽ����Ǽ��Լ� 0.1NA���� 6.02��1022����
��������
X��Y��Z��W��Ϊ������Ԫ����ԭ����������������ZWX��Ũ���ᷴӦ���л���ɫ���������������Ϊ������������ͬ���ռ���Һ���ã��ɵõ���ZWX����Һ����ZWXΪNaClO����Z��W��X�ֱ�ΪNa��Cl��O��Ԫ��Y��Wͬ�壬��YΪ��һ����ͬ��Ԫ��F�����Ԫ�������������ʵĽṹ�����ʻش�
��������������֪��X��Y��Z��W�ֱ�ΪO��F��Na��Cl����
��1��X��Y��Z��W�ֱ�ΪO��F��Na��Cl��ԭ�ӵĵ��Ӳ���Խ�٣���ԭ�Ӱ뾶ԽС��ͬһ����Ԫ���У�ԭ�Ӱ뾶����ԭ�������������С����ԭ�Ӱ뾶��Na > Cl > O > F��
�ʴ�Ϊ��O��F��Na��Cl��Na > Cl > O > F��
��2��ͬ������ϵ���Ԫ�طǽ��������μ�������̬�⻯����ȶ������μ������ǽ����ԣ�O>S����̬�⻯����ȶ��ԣ�H2O����H2S����H2OΪ������Һ��H2SΪ���ᣬ�����ԱȽϣ�H2OС��H2S��
�ʴ�Ϊ�����ڣ�С�ڣ�
��3��Na2O2Ϊ���ӻ���������Ӽ���Ǽ��Թ��ۼ��������ʽΪ��![]() ������ˮ��Ӧ�Ļ�ѧ����ʽΪ�� 2Na2O2��2H2O��4NaOH+O2����2 Na2O2
������ˮ��Ӧ�Ļ�ѧ����ʽΪ�� 2Na2O2��2H2O��4NaOH+O2����2 Na2O2 ![]() 2e ����0.1 mol��������ˮ��Ӧת�Ƶ�����Ϊ0.1NA���� 6.02��1022������
2e ����0.1 mol��������ˮ��Ӧת�Ƶ�����Ϊ0.1NA���� 6.02��1022������
�ʴ�Ϊ��![]() ��0.1NA���� 6.02��1022������
��0.1NA���� 6.02��1022������

����Ŀ�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±�:
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
T | ��������ˮ���ҷ�Ӧ,������Һ�������� |
X | L��p��������s��������2�� |
Y | ��������Ԫ�صļ������а뾶��С |
Z | L��������δ�ɶԵ��� |
(1)д��Ԫ��X�����ӽṹʾ��ͼ____________��
(2)д��YԪ������������ˮ������NaOH��Һ��Ӧ�����ӷ���ʽ______________
(3)д��Y�ļ۵����Ų�ʽ_________________________��
(4)Ԫ��T����Ԫ�����,�ǽ����Խ�ǿ����_______(��Ԫ�ط��ű�ʾ),���б�����������֤����һ��ʵ����_______(����ĸ����)��
A.��̬�⻯��Ļӷ��� B.��Ԫ�صĵ縺��
C.����������� D.�⻯����X��H���ļ���(X����T��Cl����Ԫ��)
(5)̽Ѱ���ʵ����ʲ�������ѧϰ����Ҫ����֮һ��T��X��Y��Z����Ԫ�صĵ����л�ѧ�������Բ�ͬ���������ֵ��ʵ���___(��Ԫ�ط���)