��Ŀ����
����ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��.SO2��2H2O��I2=H2SO4��2HI
��.2HI H2����I2
H2����I2
��.2H2SO4=2SO2��O2����2H2O
(1)����������Ӧ�������ж���ȷ���� ��
a����Ӧ�����ڳ����½���
b����Ӧ����SO2�����Ա�HIǿ
c��ѭ���������貹��H2O
d��ѭ�������в���1 mol O2��ͬʱ����1 mol H2
(2)һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2�����ʵ�����ʱIP��ı仯��ͼ��ʾ��
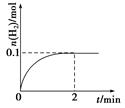
��0��2 min�ڵ�ƽ����Ӧ����v(HI)�� ��
����ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������ ��ԭ����2����
a��HI��ƽ��Ũ��
b���ﵽƽ���ʱ��
c��ƽ��ʱH2���������
(3)ʵ������Zn��ϡ������ȡH2���������������й����Լ��е� ������H2�����ʽ�����
a��NaNO3 b��CuSO4 c��Na2SO4 d��NaHSO3
��.SO2��2H2O��I2=H2SO4��2HI
��.2HI
 H2����I2
H2����I2��.2H2SO4=2SO2��O2����2H2O
(1)����������Ӧ�������ж���ȷ���� ��
a����Ӧ�����ڳ����½���
b����Ӧ����SO2�����Ա�HIǿ
c��ѭ���������貹��H2O
d��ѭ�������в���1 mol O2��ͬʱ����1 mol H2
(2)һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2�����ʵ�����ʱIP��ı仯��ͼ��ʾ��

��0��2 min�ڵ�ƽ����Ӧ����v(HI)�� ��
����ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������ ��ԭ����2����
a��HI��ƽ��Ũ��
b���ﵽƽ���ʱ��
c��ƽ��ʱH2���������
(3)ʵ������Zn��ϡ������ȡH2���������������й����Լ��е� ������H2�����ʽ�����
a��NaNO3 b��CuSO4 c��Na2SO4 d��NaHSO3
(1)c��(2)0.1 mol��L��1��min��1��64��a��(3)b
(1)H2SO4�е�ϸߣ��ڳ����²������ֽ⣻��Ӧ����SO2�Ļ�ԭ�Ա�HIǿ��ѭ��������H2O�ֽ�������H2��O2���貹�䣻ѭ�������в���1 mol O2ͬʱ����2 mol H2��c��ȷ��
(2)��2HI(g)�� H2(g)������I2(g)
H2(g)������I2(g)
��ʼ��1 mol��L��1��������0����������0
ƽ�⣺0.8 mol��L��1��0.1 mol��L��1��0.1 mol��L��1
v(HI)��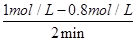 ��0.1 mol��L��1��min��1
��0.1 mol��L��1��min��1
���¶��£���ʼ����HI(g)�����ʵ�����ԭ����2����ƽ�ⳣ�����䣬HI��H2��I2ƽ��Ũ�Ⱦ�Ϊԭ����2������ʼŨ�ȱ��Ӧ���ʼӿ죬HI��H2��I2��������������䣬��ѡa��
(3)a��c�Է�Ӧ��Ӱ�죬b��Zn�û���Cu���γ�CuZnԭ��أ���Ӧ��������dʹ��Ӧ���ʱ�����
(2)��2HI(g)��
 H2(g)������I2(g)
H2(g)������I2(g)��ʼ��1 mol��L��1��������0����������0
ƽ�⣺0.8 mol��L��1��0.1 mol��L��1��0.1 mol��L��1
v(HI)��
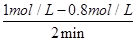 ��0.1 mol��L��1��min��1
��0.1 mol��L��1��min��1���¶��£���ʼ����HI(g)�����ʵ�����ԭ����2����ƽ�ⳣ�����䣬HI��H2��I2ƽ��Ũ�Ⱦ�Ϊԭ����2������ʼŨ�ȱ��Ӧ���ʼӿ죬HI��H2��I2��������������䣬��ѡa��
(3)a��c�Է�Ӧ��Ӱ�죬b��Zn�û���Cu���γ�CuZnԭ��أ���Ӧ��������dʹ��Ӧ���ʱ�����

��ϰ��ϵ�д�
�����Ŀ
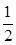 O2��g��
O2��g�� SO3��g����H����98 kJ��mol��1��
SO3��g����H����98 kJ��mol��1��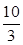 �����ڴ��¶��£���100 L�ĺ����ܱ������У�����3.0 mol
�����ڴ��¶��£���100 L�ĺ����ܱ������У�����3.0 mol



 Ni(CO)4(g)��H1��0
Ni(CO)4(g)��H1��0 Ni(S)+4CO(g) ��H2
Ni(S)+4CO(g) ��H2 
 Ni(OH)2+M ��س��ʱ�������ĵ缫��ӦʽΪ ����س��ʱ�����Ϸ��� �����������ԭ������Ӧ
Ni(OH)2+M ��س��ʱ�������ĵ缫��ӦʽΪ ����س��ʱ�����Ϸ��� �����������ԭ������Ӧ N2��4CO2�ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ��Ӧ
N2��4CO2�ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ��Ӧ 2SO3��һ��ʱ���,SO3��Ũ��������0.4 mol��L-1,�����ʱ������O2��ʾ�ķ�Ӧ����Ϊ0.04 mol��(L��s)-1,�����ʱ��Ϊ(����)
2SO3��һ��ʱ���,SO3��Ũ��������0.4 mol��L-1,�����ʱ������O2��ʾ�ķ�Ӧ����Ϊ0.04 mol��(L��s)-1,�����ʱ��Ϊ(����)