��Ŀ����
��372Kʱ����0.5molN2O4ͨ�����Ϊ5L������ܱ������У��������ֺ���ɫ����Ӧ���е�2sʱ��NO2��Ũ��Ϊ 0.02mol��L��1����60sʱ����ϵ�Ѵ�ƽ�⣬��ʱ������ѹǿΪ��ʼʱ��1.6��������˵����ȷ���ǣ� ��
| A��ǰ2s��N2O4��Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ0.01mol��L��1��s��1 |
| B����2sʱ��ϵ��ѹǿΪ��ʼʱ��1.1�� |
| C����ƽ��ʱ��ϵ�ں���N2O40.25mol |
| D��ƽ��ʱ��N2O4��ת����Ϊ40% |
B
N2O4��NO2��������ת����ϵN2O4 2NO2��ǰ2sʱ
2NO2��ǰ2sʱ
N2O4�� ��2NO2
��2NO2
��ʼ��mol�� 0.5 0
��Ӧ��mol�� 0.05 0.02��5
2sʱ��mol�� 0.5��0.05 0.02��5
v��N2O4���� ��0.005mol��L��1��s��1
��0.005mol��L��1��s��1
�����ܵ����ʵ���Ϊ0.5mol��0.05mol��0.02mol��L��1��5L��0.55mol��
2sʱ�뿪ʼ��ѹǿ֮��Ϊp��2s����p��ʼ����0.55��0.5��1.1��1��
60s�ﵽƽ��ʱ����μӷ�Ӧ��N2O4�����ʵ���Ϊx������
��N2O4 2NO2
2NO2
��ʼ��mol�� 0.5 0
��Ӧ��mol�� x 2x
ƽ�⣨mol�� 0.5��x 2x
ƽ��ʱ�������ܵ����ʵ���Ϊ0.5mol��x��2x��0.5mol��x�������� ��1.6�����x��0.3mol��
��1.6�����x��0.3mol��
ƽ����ϵ�к�0.2molN2O4��N2O4��ת����Ϊ ��100%��60%��
��100%��60%��
 2NO2��ǰ2sʱ
2NO2��ǰ2sʱN2O4��
 ��2NO2
��2NO2��ʼ��mol�� 0.5 0
��Ӧ��mol�� 0.05 0.02��5
2sʱ��mol�� 0.5��0.05 0.02��5
v��N2O4����
 ��0.005mol��L��1��s��1
��0.005mol��L��1��s��1�����ܵ����ʵ���Ϊ0.5mol��0.05mol��0.02mol��L��1��5L��0.55mol��
2sʱ�뿪ʼ��ѹǿ֮��Ϊp��2s����p��ʼ����0.55��0.5��1.1��1��
60s�ﵽƽ��ʱ����μӷ�Ӧ��N2O4�����ʵ���Ϊx������
��N2O4
 2NO2
2NO2��ʼ��mol�� 0.5 0
��Ӧ��mol�� x 2x
ƽ�⣨mol�� 0.5��x 2x
ƽ��ʱ�������ܵ����ʵ���Ϊ0.5mol��x��2x��0.5mol��x��������
 ��1.6�����x��0.3mol��
��1.6�����x��0.3mol��ƽ����ϵ�к�0.2molN2O4��N2O4��ת����Ϊ
 ��100%��60%��
��100%��60%��
��ϰ��ϵ�д�
 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
�����Ŀ
 H2����I2
H2����I2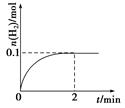
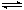 CH3OH(g) ��H=akJ/mol���ݻ��̶���2L�ܱ������г���2mol CO(g)��4molH2(g)������Ӧ���ⶨ�ڲ�ͬ�¶ȡ���ͬʱ����CO��ת�������±���
CH3OH(g) ��H=akJ/mol���ݻ��̶���2L�ܱ������г���2mol CO(g)��4molH2(g)������Ӧ���ⶨ�ڲ�ͬ�¶ȡ���ͬʱ����CO��ת�������±���

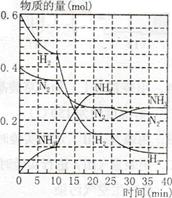
 2C(g)��D(g)����2 min��B��Ũ�ȼ���0.6 mol/L���Դ˷�Ӧ���ʵı�ʾ��ȷ����(����)
2C(g)��D(g)����2 min��B��Ũ�ȼ���0.6 mol/L���Դ˷�Ӧ���ʵı�ʾ��ȷ����(����) 2C(g)������2 s����C��Ũ��Ϊ0.6 mol/L�����м���˵����ȷ����
2C(g)������2 s����C��Ũ��Ϊ0.6 mol/L�����м���˵����ȷ����