��Ŀ����
��2011?������ģ�⣩�����������A��B��Ϊͬ���칹�壬����C��H��O����Ԫ�أ���ͬ״���£�A��B����������������ܶ���97��������C��Hԭ�Ӹ�����ͬ����C��Hԭ����������ԭ������5����
��1��A�ķ���ʽ��
��֪�����л���������ͼת����ϵ

����C�ܷ���������Ӧ��F����������������C��C��D����Է���������ͬ�IJ�ͬ���л��
��2��E�����������ŵ�������
a��C3H6O3 b��C3H8O c��C2H4 d��C2H6O2
��3��B�Ľṹ��ʽ��
 ��
��
ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ��
 ��
��
C��F��Ӧ�Ļ�ѧ����ʽ��
 ����Ӧ������
����Ӧ������
��4��ͬʱ��������Ҫ���ͬ���칹����
����A��Ϊͬ���칹�� �ڿ���ˮ�� �۱�����������ȡ�������ұ����ϵ�һ�ȴ���ֻ��1�֣�
��5��1mol������4���е�һ���л���X������4mol NaOH������Ӧ��д���˷�Ӧ�Ļ�ѧ����ʽ
 ��
��
��1��A�ķ���ʽ��
C10H10O4
C10H10O4
����֪�����л���������ͼת����ϵ

����C�ܷ���������Ӧ��F����������������C��C��D����Է���������ͬ�IJ�ͬ���л��
��2��E�����������ŵ�������
�Ȼ�
�Ȼ�
����ȫȼ��ʱ��1mol D��1mol ����ac
ac
�ĺ�������ͬ������ĸ���ţ���a��C3H6O3 b��C3H8O c��C2H4 d��C2H6O2
��3��B�Ľṹ��ʽ��


ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ��


C��F��Ӧ�Ļ�ѧ����ʽ��


������Ӧ
������Ӧ
����4��ͬʱ��������Ҫ���ͬ���칹����
3
3
�֣�����A��Ϊͬ���칹�� �ڿ���ˮ�� �۱�����������ȡ�������ұ����ϵ�һ�ȴ���ֻ��1�֣�
��5��1mol������4���е�һ���л���X������4mol NaOH������Ӧ��д���˷�Ӧ�Ļ�ѧ����ʽ


�����������������A��B��һ���б�������A��B����������������ܶ���97������֪��A��B�ķ�������194��������C��Hԭ�Ӹ�����ͬ����C��Hԭ����������ԭ������5���������ʽһ����C10H10O4����������ͬ���칹���� ��
��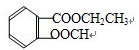 ���֣�����ȩ���������ܷ���������Ӧ��C�Ǽ��ᣬDΪ�Ҵ���F����������������C������F�Ǽ״���
���֣�����ȩ���������ܷ���������Ӧ��C�Ǽ��ᣬDΪ�Ҵ���F����������������C������F�Ǽ״���
��1��A��B����������������ܶ���97������֪��A�ķ�������194������̼�����ĸ���֮��Ĺ�ϵȷ���л����ʵķ���ʽ��
��2���������ʵķ����Լ������ŵĸ��������ȼ�պ������������ش�
��3�����ݷ���������ﺬ�б����Ľṹ�Լ�����ʽ��ˮ�������ȷ���ṹ��ʽ���������ʵ�������д��ѧ����ʽ��
��4����������ͬ�ṹ��ͬ���л���֮�以��Ϊͬ���칹�壻
��5�������л����ڼ��Ի����µ�ˮ��ԭ������д����ʽ��
 ��
��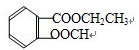 ���֣�����ȩ���������ܷ���������Ӧ��C�Ǽ��ᣬDΪ�Ҵ���F����������������C������F�Ǽ״���
���֣�����ȩ���������ܷ���������Ӧ��C�Ǽ��ᣬDΪ�Ҵ���F����������������C������F�Ǽ״�����1��A��B����������������ܶ���97������֪��A�ķ�������194������̼�����ĸ���֮��Ĺ�ϵȷ���л����ʵķ���ʽ��
��2���������ʵķ����Լ������ŵĸ��������ȼ�պ������������ش�
��3�����ݷ���������ﺬ�б����Ľṹ�Լ�����ʽ��ˮ�������ȷ���ṹ��ʽ���������ʵ�������д��ѧ����ʽ��
��4����������ͬ�ṹ��ͬ���л���֮�以��Ϊͬ���칹�壻
��5�������л����ڼ��Ի����µ�ˮ��ԭ������д����ʽ��
����⣺�����������A��B��һ���б�������A��B����������������ܶ���97������֪��A��B�ķ�������194��������C��Hԭ�Ӹ�����ͬ����C��Hԭ����������ԭ������5���������ʽһ����C10H10O4����������ͬ���칹���� ��
��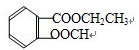 ���֣�����ȩ���������ܷ���������Ӧ��C�Ǽ��ᣬDΪ�Ҵ���F����������������C������F�Ǽ״���
���֣�����ȩ���������ܷ���������Ӧ��C�Ǽ��ᣬDΪ�Ҵ���F����������������C������F�Ǽ״���
��1��A�ķ���ʽ��C10H10O4���ʴ�Ϊ��C10H10O4��
��2������ʽΪC10H10O4�ķ�������ͬ���칹���� ��
��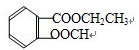 ���֣����������Ի����»ᷢ��ˮ�⣬����C�ܷ���������Ӧ��DΪ�Ҵ���F����������������C��F�Ǽ״���������Ҵ�����Է���������ͬ�IJ�ͬ���л����ȫȼ��1mol�Ҵ�����������3mol���ͱ������Լ���ϩ�ĺ���������ȵģ��ʴ�Ϊ���Ȼ���ac��
���֣����������Ի����»ᷢ��ˮ�⣬����C�ܷ���������Ӧ��DΪ�Ҵ���F����������������C��F�Ǽ״���������Ҵ�����Է���������ͬ�IJ�ͬ���л����ȫȼ��1mol�Ҵ�����������3mol���ͱ������Լ���ϩ�ĺ���������ȵģ��ʴ�Ϊ���Ȼ���ac��
��3��Bˮ����Եõ��״������Խṹ��ʽΪ�� ��ˮ�����е��Ȼ����Ժ�̼�����Ʒ�����Ӧ�����ɶ�����̼������
��ˮ�����е��Ȼ����Ժ�̼�����Ʒ�����Ӧ�����ɶ�����̼������ ���״��ͼ���֮����Է���������Ӧ����������
���״��ͼ���֮����Է���������Ӧ����������  ���÷�Ӧ����������Ӧ���ʴ�Ϊ��
���÷�Ӧ����������Ӧ���ʴ�Ϊ�� ��
�� ��
�� ��������Ӧ��
��������Ӧ��
��4������ˮ��˵������������������������ȡ�������ұ����ϵ�һ�ȴ���ֻ��1�֣�֤�������ϵ���ԭ�ӵ�Ч�������Ľṹ�У� ��
��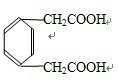 ��
�� ���ʴ�Ϊ��3��
���ʴ�Ϊ��3��
��5���ڼ��Ի����£�����ˮ�ⷽ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
 ��
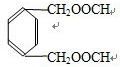
��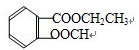 ���֣�����ȩ���������ܷ���������Ӧ��C�Ǽ��ᣬDΪ�Ҵ���F����������������C������F�Ǽ״���
���֣�����ȩ���������ܷ���������Ӧ��C�Ǽ��ᣬDΪ�Ҵ���F����������������C������F�Ǽ״�����1��A�ķ���ʽ��C10H10O4���ʴ�Ϊ��C10H10O4��
��2������ʽΪC10H10O4�ķ�������ͬ���칹����
 ��
��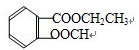 ���֣����������Ի����»ᷢ��ˮ�⣬����C�ܷ���������Ӧ��DΪ�Ҵ���F����������������C��F�Ǽ״���������Ҵ�����Է���������ͬ�IJ�ͬ���л����ȫȼ��1mol�Ҵ�����������3mol���ͱ������Լ���ϩ�ĺ���������ȵģ��ʴ�Ϊ���Ȼ���ac��
���֣����������Ի����»ᷢ��ˮ�⣬����C�ܷ���������Ӧ��DΪ�Ҵ���F����������������C��F�Ǽ״���������Ҵ�����Է���������ͬ�IJ�ͬ���л����ȫȼ��1mol�Ҵ�����������3mol���ͱ������Լ���ϩ�ĺ���������ȵģ��ʴ�Ϊ���Ȼ���ac����3��Bˮ����Եõ��״������Խṹ��ʽΪ��
 ��ˮ�����е��Ȼ����Ժ�̼�����Ʒ�����Ӧ�����ɶ�����̼������
��ˮ�����е��Ȼ����Ժ�̼�����Ʒ�����Ӧ�����ɶ�����̼������ ���״��ͼ���֮����Է���������Ӧ����������
���״��ͼ���֮����Է���������Ӧ����������  ���÷�Ӧ����������Ӧ���ʴ�Ϊ��
���÷�Ӧ����������Ӧ���ʴ�Ϊ�� ��
�� ��
�� ��������Ӧ��
��������Ӧ�� ��4������ˮ��˵������������������������ȡ�������ұ����ϵ�һ�ȴ���ֻ��1�֣�֤�������ϵ���ԭ�ӵ�Ч�������Ľṹ�У�
 ��
�� ��
�� ���ʴ�Ϊ��3��
���ʴ�Ϊ��3�� ��5���ڼ��Ի����£�����ˮ�ⷽ��ʽΪ��
 ��
���ʴ�Ϊ��
 ��
��������������һ���й��л���Ľṹ������֪ʶ���ۺ��ƶϣ�����ѧ�������ͽ��������������ۺ���ǿ���Ѷȴ�

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ
 ��2011?������ģ�⣩һ�������£���һ������A��B��C��D�������ʣ������ܱ������з������·�Ӧ��
��2011?������ģ�⣩һ�������£���һ������A��B��C��D�������ʣ������ܱ������з������·�Ӧ�� p C��g��+q D��g���ﵽƽ����B��Ũ��Ϊ0.5
p C��g��+q D��g���ﵽƽ����B��Ũ��Ϊ0.5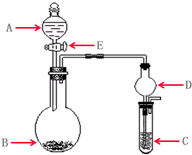 ��2011?������ģ�⣩ʵ������Ҫ����ijЩ����ʱ��ͨ��ʹ�ÿ��ٵķ����Ʊ������м���ʵ��ɿ�����ȡʵ����������������壬�������������ʵ�飮��ʵ��װ����ͼ��ʾ��
��2011?������ģ�⣩ʵ������Ҫ����ijЩ����ʱ��ͨ��ʹ�ÿ��ٵķ����Ʊ������м���ʵ��ɿ�����ȡʵ����������������壬�������������ʵ�飮��ʵ��װ����ͼ��ʾ��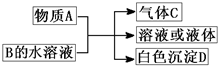 ��2011?������ģ�⣩A��B��C��D��Ϊ��ѧ��ѧ�������ʣ�����֮��ķ�Ӧ��ϵ��ͼ��ʾ��
��2011?������ģ�⣩A��B��C��D��Ϊ��ѧ��ѧ�������ʣ�����֮��ķ�Ӧ��ϵ��ͼ��ʾ��