��Ŀ����
ﯣ�Zr����һ����Ҫ��ϡ��Ԫ�ء�
��1�����Ӣʯ��ZrSiO4������ȡZrOC12-8H2O
����̼�����Ӣʯ��̼��ﯵĻ�ѧ����ʽΪ��ZrSiO4��3C ZrC��SiO2��2CO�����÷�Ӧ���������뻹ԭ����������Ϊ ��
ZrC��SiO2��2CO�����÷�Ӧ���������뻹ԭ����������Ϊ ��
�����ռ���ZrC��ȡNa2ZrO3����ɸ÷�Ӧ�Ļ�ѧ����ʽ��
��ZrC+��NaOH+��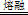 ��Na2ZrO3+��CO2+��H2O
��Na2ZrO3+��CO2+��H2O
�ۼ���������������ɵ�ZrOC12��Һ��ͨ�������ᾧ�Ӹ���Һ�л��ZrOC12-8H2O��������Ũ���������ȣ�ԭ���� ��
��2��ZrOC12-8H2O���ж�����;����ZrOC12-8H2O��YC13�Ʊ�Y2O3����ZrO2���Ϸ�����������£�

�ٹ�����ʱ������Zr(OH)4�Ļ�ѧ����ʽΪ ��
�ڵ�������ǡ����ȫʱ����Һ��c(Zr4��)= ��
����֪Ksp[Zr(OH)4]=6.4��10��49��Ksp[Y(OH)3]=8.0��10��23����Һ������Ũ��Ϊ1��10��5mol?L��1ʱ������Ϊ������ȫ��
�۽��Ƶõ�һ�ָ��Ϸ�������ˮ���������ƽ��ֱ��Ϊ30 nm���÷�ɢϵ���� ��
��1�����Ӣʯ��ZrSiO4������ȡZrOC12-8H2O
����̼�����Ӣʯ��̼��ﯵĻ�ѧ����ʽΪ��ZrSiO4��3C
 ZrC��SiO2��2CO�����÷�Ӧ���������뻹ԭ����������Ϊ ��
ZrC��SiO2��2CO�����÷�Ӧ���������뻹ԭ����������Ϊ �������ռ���ZrC��ȡNa2ZrO3����ɸ÷�Ӧ�Ļ�ѧ����ʽ��
��ZrC+��NaOH+��
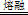 ��Na2ZrO3+��CO2+��H2O
��Na2ZrO3+��CO2+��H2O�ۼ���������������ɵ�ZrOC12��Һ��ͨ�������ᾧ�Ӹ���Һ�л��ZrOC12-8H2O��������Ũ���������ȣ�ԭ���� ��
��2��ZrOC12-8H2O���ж�����;����ZrOC12-8H2O��YC13�Ʊ�Y2O3����ZrO2���Ϸ�����������£�

�ٹ�����ʱ������Zr(OH)4�Ļ�ѧ����ʽΪ ��
�ڵ�������ǡ����ȫʱ����Һ��c(Zr4��)= ��
����֪Ksp[Zr(OH)4]=6.4��10��49��Ksp[Y(OH)3]=8.0��10��23����Һ������Ũ��Ϊ1��10��5mol?L��1ʱ������Ϊ������ȫ��
�۽��Ƶõ�һ�ָ��Ϸ�������ˮ���������ƽ��ֱ��Ϊ30 nm���÷�ɢϵ���� ��
��15�֣���1����1��2��2�֣� ��ZrC+2NaOH+2O2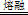 Na2ZrO3+CO2+H2O��3�֣�
Na2ZrO3+CO2+H2O��3�֣�
�۷�ֹZrO2+��ˮ�⣨2�֣�
��2����ZrOCl2+2NH3?H2O+H2O=Zr(OH)4��+2NH4Cl��3�֣�
��4.0��10��26mol?L��1��3�֣�
�۽��壨2�֣�
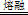 Na2ZrO3+CO2+H2O��3�֣�
Na2ZrO3+CO2+H2O��3�֣��۷�ֹZrO2+��ˮ�⣨2�֣�
��2����ZrOCl2+2NH3?H2O+H2O=Zr(OH)4��+2NH4Cl��3�֣�
��4.0��10��26mol?L��1��3�֣�
�۽��壨2�֣�
�����������1����̼Ԫ�صĻ��ϼۼ������ֽ��ͣ�C�������������ǻ�ԭ����ZrC�ǻ�ԭ���CO����������һ�ԭ������������������ʵ���֮��Ϊ1��2�����������뻹ԭ�������ʵ���������֮��Ϊ1��2����̼Ԫ���ɡ�4����Ϊ+4�ۣ���������������Ԫ����0�۽�Ϊ��2�ۣ����ݻ��ϼ�����������ȡ�ԭ���غ���ƽ�ɵã�ZrC+2NaOH+2O2
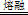 Na2ZrO3+CO2+H2O���������������������ȵ���ҪĿ�������������ˮ����ֹZrO2+��ˮ�⣻��2���ٶ�ͼ��֪���������������ֽⷴӦ����ӦʽΪZrOCl2+2NH3?H2O+H2O=Zr(OH)4��+2NH4Cl���ڵ�Y3+������ȫʱ����Y(OH)3(s)
Na2ZrO3+CO2+H2O���������������������ȵ���ҪĿ�������������ˮ����ֹZrO2+��ˮ�⣻��2���ٶ�ͼ��֪���������������ֽⷴӦ����ӦʽΪZrOCl2+2NH3?H2O+H2O=Zr(OH)4��+2NH4Cl���ڵ�Y3+������ȫʱ����Y(OH)3(s) Y3+(aq)+3OH��(aq)��֪��c(OH��)=
Y3+(aq)+3OH��(aq)��֪��c(OH��)=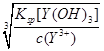 =
=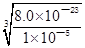 =2.0��10��6mol?L��1����Zr(OH)4(s)
=2.0��10��6mol?L��1����Zr(OH)4(s) Zr4+(aq)+4OH��(aq)��֪��c(Zr4+)=
Zr4+(aq)+4OH��(aq)��֪��c(Zr4+)=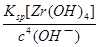 =
=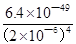 mol?L��1=4.0��10��26mol?L��1����1nm<30nm<100nm����÷�ɢϵ���ڽ��塣
mol?L��1=4.0��10��26mol?L��1����1nm<30nm<100nm����÷�ɢϵ���ڽ��塣
��ϰ��ϵ�д�
�����Ŀ
 Cu+H2O����Ӧ����ͼ�е����� ������ţ������з�Ӧ����������7���� ��
Cu+H2O����Ӧ����ͼ�е����� ������ţ������з�Ӧ����������7���� ��
 2H2O
2H2O  Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2�� N2+6NH4Cl
N2+6NH4Cl
 BrO��
BrO��

