��Ŀ����
ijͭ��ʯ������ͭ��������ͭ�������������ʹ�����ʯ��SiO2��,�ֲ���������ӿ�ʯ����ȡͭ��������ͼ���£�

��֪�� �ٵ���ʯ����������������̫��ʱ������������������Ļ��Һ����ͭ���ڷ���ȡ���ˮ��������ͭ��Һ��Cu2+Ũ��ԼΪ50g/L���ش��������⣺
��1����ʯ��ϡ�������������������ͭ�����ķ�ӦΪ��Cu2O+2H+===Cu2++Cu+H2O����д���ù����з�������һ��������ԭ��Ӧ�����ӷ���ʽ�� ��
��2��д���ö��Ե缫���ˮ��ĵ���ܷ�Ӧ����ʽ�� ��
��3��ѭ���з���ȡ��B����Ҫ�ɷ��� ��
��4��ijͭ��ʯ��Ʒ�У�������������ͭ����������������ʯ�������ʡ�ȡ�ÿ�ʯ��Ʒ200.0g����100mL1.0mol?L��1H2SO4��Һ��ȡ�������10mL 1.0mol?L��1 Fe2(SO4)3��Һ����ʹͭȫ����������ȡҺ����ֵ���ɵõ� 6.4gCu����ͭ��ʯ��Ʒ��������ͭ��������������������

��֪�� �ٵ���ʯ����������������̫��ʱ������������������Ļ��Һ����ͭ���ڷ���ȡ���ˮ��������ͭ��Һ��Cu2+Ũ��ԼΪ50g/L���ش��������⣺
��1����ʯ��ϡ�������������������ͭ�����ķ�ӦΪ��Cu2O+2H+===Cu2++Cu+H2O����д���ù����з�������һ��������ԭ��Ӧ�����ӷ���ʽ�� ��
��2��д���ö��Ե缫���ˮ��ĵ���ܷ�Ӧ����ʽ�� ��
��3��ѭ���з���ȡ��B����Ҫ�ɷ��� ��
��4��ijͭ��ʯ��Ʒ�У�������������ͭ����������������ʯ�������ʡ�ȡ�ÿ�ʯ��Ʒ200.0g����100mL1.0mol?L��1H2SO4��Һ��ȡ�������10mL 1.0mol?L��1 Fe2(SO4)3��Һ����ʹͭȫ����������ȡҺ����ֵ���ɵõ� 6.4gCu����ͭ��ʯ��Ʒ��������ͭ��������������������
��12�֣���1��Cu+2Fe3+��2Fe2++ Cu2+��2�֣�
��2��2CuSO4 + 2H2O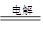 O2��+ 2Cu + 2H2SO4��2�֣�
O2��+ 2Cu + 2H2SO4��2�֣�
��3��H2SO4��2�֣� ��4��Cu2O��3.6% ��3�֣� Fe2O3��3.2%��3�֣�
��2��2CuSO4 + 2H2O
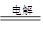 O2��+ 2Cu + 2H2SO4��2�֣�
O2��+ 2Cu + 2H2SO4��2�֣���3��H2SO4��2�֣� ��4��Cu2O��3.6% ��3�֣� Fe2O3��3.2%��3�֣�
�����������1������ͭ�����������������Ժ�ǿ�ᷢ����Ӧ�����κ�ˮ��CuO+2H+��Cu2++H2O��Fe2O3+6H+��2Fe3++3H2O��������ͭ��������֮����Է���������ԭ��Ӧ����Cu+2Fe3+��2Fe2++Cu2+��
��2�����Ե缫�������ͭ�Ļ�ѧ����ʽ��2CuSO4 + 2H2O
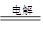 O2��+ 2Cu + 2H2SO4��
O2��+ 2Cu + 2H2SO4����3����������ͭ���������ܼ��У�����ѭ���з���ȡ��B����Ҫ�ɷ���ϡ���ᡣ
��4���������ɵ�ͭ��������6.4g�����ʵ�����0.1mol�������ԭ���غ��֪������ͭ�����ʵ�����0.05mol������ͭ��ʯ��Ʒ��������ͭ����������
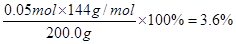 ��
�����ݷ���ʽCu2O+2H+��Cu2++Cu+H2O����Ӧ�����ɵ�ͭ��0.05mol�������Cu+2Fe3+��2Fe2++ Cu2+��֪��ͭ��Ӧ����������0.05mol��������0.01L��1.0mol/L��0.01mol���������������ģ�����������ϡ���ᷴӦ���ɵ���������0.04mol���������������ʵ�����0.04mol������������������������
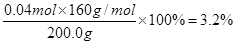 ��
�������������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ���������У����ض�ѧ������֪ʶ�Ĺ�����ѵ����ͬʱҲע�ض�ѧ�����������������ͷ���ָ��������������ѧ������˼ά�����ͷ�ɢ˼ά����������������һ���ۺ��Խ�ǿ�����⣬��Ԫ�ؼ����������ʺ�������������������ѧ�����֪ʶ���������ɿ���ѧ���Ի�ѧ֪ʶ������̶ȣ�����Ҫ��������ѧ�����ۺϷ���������˼ά������

��ϰ��ϵ�д�
 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ
 ZrC��SiO2��2CO�����÷�Ӧ���������뻹ԭ����������Ϊ ��
ZrC��SiO2��2CO�����÷�Ӧ���������뻹ԭ����������Ϊ �� ��Na2ZrO3+��CO2+��H2O
��Na2ZrO3+��CO2+��H2O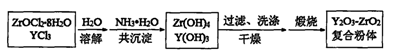
 3HNCO��8HNCO+6NO2
3HNCO��8HNCO+6NO2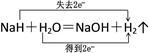
 ������һ�������¿���Mn2+��������Ϊ
������һ�������¿���Mn2+��������Ϊ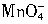 ������Ӧ��
������Ӧ�� ���ӱ�Ϊ
���ӱ�Ϊ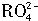 ���ӣ�
���ӣ�