��Ŀ����
���ߵ���H5IO6����MnSO4��Һ�п�ʹ��Һ���Ϻ�ɫ����֪H5IO6�ڷ�Ӧ�з������¹��̣�H5IO6��HIO3�����������գ�
��1�����÷�Ӧ������������ԭ������ƽ���ϵ��������ȷλ�á�
��2������ͼ�б�������ת�Ƶķ������Ŀ��
��3������H5IO6��Һ�м����������������ʣ��ܽ���Ԫ�ػ�ԭ�ɵ����ӵ���____��ѡ����ţ���
a. ���� b. ���� c. �廯�� d. ��������
��4������1mol H5IO6����Һ�м�������Ĺ���������Һ���ټ��������Һ����Һ������ͬʱ�д��������������д���˷�Ӧ�Ļ�ѧ����ʽ��________________________________���˹��������ٿɵõ�����_________L����״̬�£���
��1�����÷�Ӧ������������ԭ������ƽ���ϵ��������ȷλ�á�
��2������ͼ�б�������ת�Ƶķ������Ŀ��
��3������H5IO6��Һ�м����������������ʣ��ܽ���Ԫ�ػ�ԭ�ɵ����ӵ���____��ѡ����ţ���
a. ���� b. ���� c. �廯�� d. ��������
��4������1mol H5IO6����Һ�м�������Ĺ���������Һ���ټ��������Һ����Һ������ͬʱ�д��������������д���˷�Ӧ�Ļ�ѧ����ʽ��________________________________���˹��������ٿɵõ�����_________L����״̬�£���
�����8�֣���1������2������3�֣�����1�֡���ƽ1�֡�������Ŀ1�֣�

��3��b��1�֣���
��4��2H5IO6 + 7H2O2 �� I2 + 12H2O + 7O2����2�֣���78.4L��2�֣�

��3��b��1�֣���
��4��2H5IO6 + 7H2O2 �� I2 + 12H2O + 7O2����2�֣���78.4L��2�֣�
�����������1������2���ߵ���H5IO6����MnSO4��Һ�п�ʹ��Һ���Ϻ�ɫ����˵����Ӧ���и���������ɣ���˸ߵ��������������������ǻ�ԭ��������IԪ�صĻ��ϼ۴ӣ�7�۽��͵���5�ۣ��õ�2�����ӡ�MnԪ�صĻ��ϼ۴ӣ�2�����ߵ���7�ۣ�ʧȥ5�����ӣ����Ը��ݵ��ӵ�ʧ�غ��֪���������뻹ԭ�������ʵ���֮����5:2����˵���ת�Ƶķ������ĿΪ
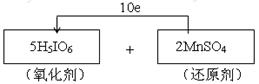 ��
����3�������������������Լ������ӵ������Ծ�ǿ�ڵ��ʵ�ģ�����ѡ��acd�����ܽ��ߵ��ỹԭΪ�����ӡ����ʵ��������ǿ��S�ģ����H2S���Ѹߵ��ỹԭΪ�����ӣ���ѡb��
��4����1mol H5IO6����Һ�м�������Ĺ���������Һ���ټ��������Һ����Һ��������˵���е��ʵ����ɡ�ͬʱ�д������������������Ӧ�������������Է�Ӧ�Ļ�ѧ����ʽΪ2H5IO6 + 7H2O2 �� I2 + 12H2O + 7O2�����������������ʵ�����3.5mol���ڱ�״���µ������3.5mol��22.4L/mol��78.4L��

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ
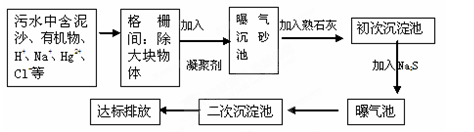
 ZrC��SiO2��2CO�����÷�Ӧ���������뻹ԭ����������Ϊ ��
ZrC��SiO2��2CO�����÷�Ӧ���������뻹ԭ����������Ϊ �� ��Na2ZrO3+��CO2+��H2O
��Na2ZrO3+��CO2+��H2O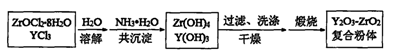
 3HNCO��8HNCO+6NO2
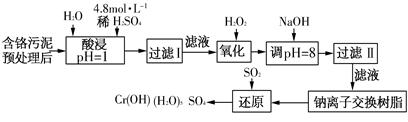
3HNCO��8HNCO+6NO2 ������һ�������¿���Mn2+��������Ϊ
������һ�������¿���Mn2+��������Ϊ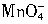 ������Ӧ��
������Ӧ�� ���ӱ�Ϊ
���ӱ�Ϊ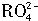 ���ӣ�
���ӣ�